Synthetic method for milbemycin oxime
A synthesis method and technology of mirbe oxime are applied in the synthesis field of semi-synthetic macrolide anthelmintic drug mirbe oxime, can solve the problems of toxic chromium trioxide, heavy metal pollution, many side reactions and the like, and achieve low cost, Less side effects and milder effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
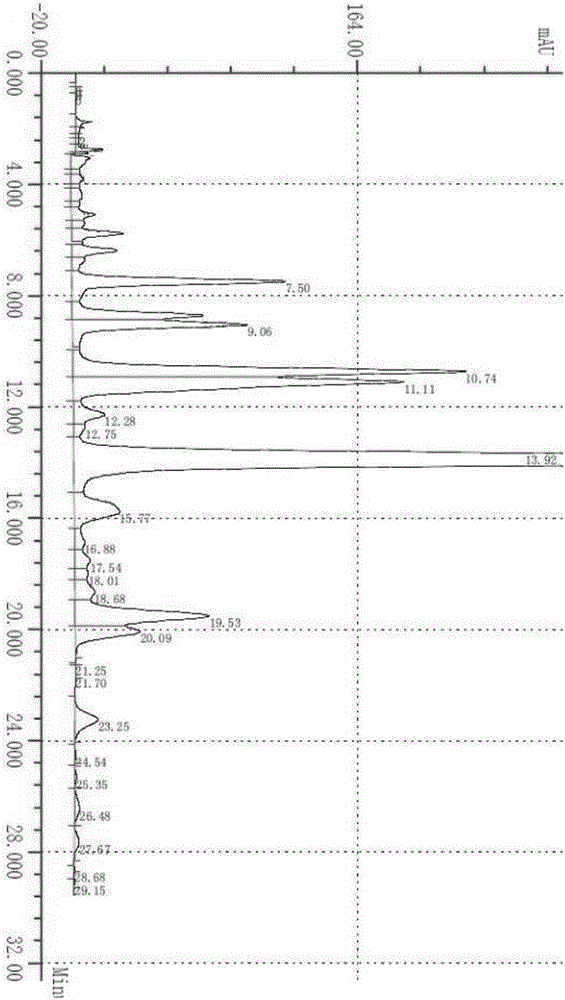
[0023] Contain 5Kg milbemycin (C 31 h 43 o 7 or C 32 h 45 o 7 ) of the raw material 24.7Kg is placed in the reactor, and the spectrogram obtained by the milbemycin liquid chromatography detection used in the present embodiment is as follows figure 1 as shown, figure 1 Among them, the peak at 11.11min is Milbemycin A 3 , the peak at 13.92min is Milbemycin A 4 .
[0024] Add 300g of 2,2,6,6-tetramethylpyridine-N-oxyl radical into the reaction kettle, add 100L of dichloromethane to dissolve, set the temperature of the refrigeration equipment to 5°C, turn on the refrigeration, and turn on the stirring. Weigh 200g of sodium bromide, dissolve it in 1000ml of deionized water and add it to the reaction solution;
[0025] (2), take by weighing 3.57Kg sodium bicarbonate and 11.76Kg sodium carbonate, add 100L water to dissolve, add 81Kg20% sodium hypochlorite solution, stir well, adjust pH to 10 ± 0.5;
[0026] (3), the oxidant solution is divided into 5 batches and added dropw...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com