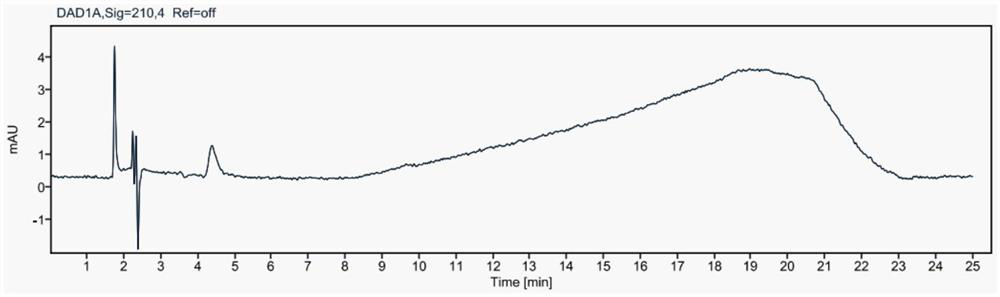
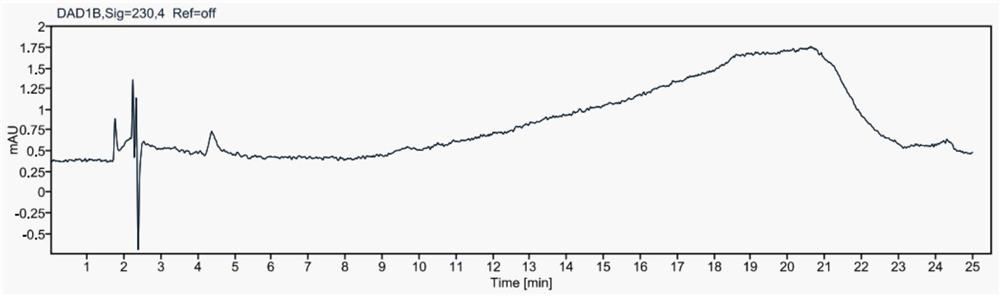
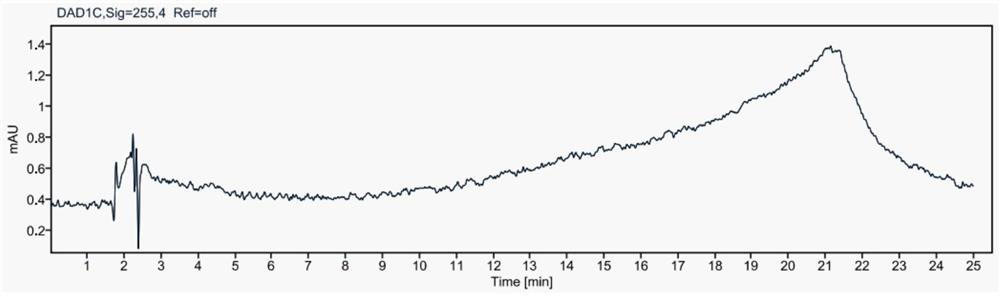
Method for simultaneously detecting contents of praziquantel, clofenoxine and milbemycin oxime
A technology of milbexime and praziquantel, which is applied in the field of chemical analysis, can solve problems such as undisclosed detection methods of the content, and achieve the effects of simple detection methods, cost reduction, and time saving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] Example 1: System Suitability
[0100] Chromatography program
[0101] Do system suitability test according to the requirements of the analysis method. After the system is balanced, take 20 μL of the reference substance solution, inject it into the liquid chromatograph, and record the chromatogram. Advanced 5 needles Std-1, calculate the relative standard deviation (RSD), then enter 2 needles Std-2, use Std-1 to calculate the accuracy of Std-2, as shown in Table 7.
[0102] Table 7
[0103]
[0104]
[0105] The formula for calculating the recovery rate of Std-2 with Std-1:
[0106]
[0107] A Std-1 =The average value of the peak area of each component in the Std-1 chromatogram.
[0108] A Std-2 =The average value of the peak area of each component in the Std-2 chromatogram.
[0109] C Std-1 =Concentration of each component in Std-1.
[0110] C Std-2 =Concentration of each component in Std-2.
[0111] The system suitability test results are shown...
Embodiment 2
[0122] Example 2: Specificity
[0123] Specificity will be confirmed by the interference of the blank and the interference of degraded impurities.
[0124] Blank solution (diluent): Inject acetonitrile directly.
[0125] Sample: Weigh the raw material of praziquantel (source: Hebei Jiayi Pharmaceutical Co., Ltd., batch number: JYC-200402), the new raw material of chlorophene (source: Zhejiang Haisheng, batch number: N181101), the raw material of milbexime (source: Zhejiang Hisun Pharmaceutical Co., Ltd., batch number: 4037-A180405U) constituted a mixture with a similar weight of 14.2 mg as a sample for the destruction test.
[0126] Acid degradation: Weigh the sample, put it in a 100mL measuring bottle, add 5.0mL, 1mol / L hydrochloric acid solution, leave it at room temperature for 5min, adjust the pH value to 7.0 with 1mol / L sodium hydroxide solution, cool to room temperature, Dilute to the mark with diluent, shake well, that is.
[0127] Alkali degradation: Weigh the sampl...
Embodiment 3
[0135] Example 3: Linear
[0136] The linearity studies of praziquantel, clofenacine and milbexime in the assay ranged from 60-140% concentration. Carry out the linearity study test as follows.
[0137] Test Procedures and Results Processing
[0138] With the concentration (μg / mL) as the abscissa and the peak area as the ordinate, draw the standard curve y=ax+b.
[0139] Calculate the correlation coefficient r of the standard curve.
[0140] Calculate the linear deviation Bias%: that is, the intercept b is relative to the peak area y at 100% concentration 100% percentage.
[0141]
[0142] Calculate the mean response factor (MRF):
[0143]
[0144] The linearity test results are shown in Table 13 to Table 15, Figure 9 to Figure 11 shown.
[0145] Table 13
[0146]
[0147]
[0148] Table 14
[0149]
[0150]
[0151] Table 15
[0152]
[0153] In summary, it can be seen that the repeatability RSD of three injections at each concentration n=3 Al...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com