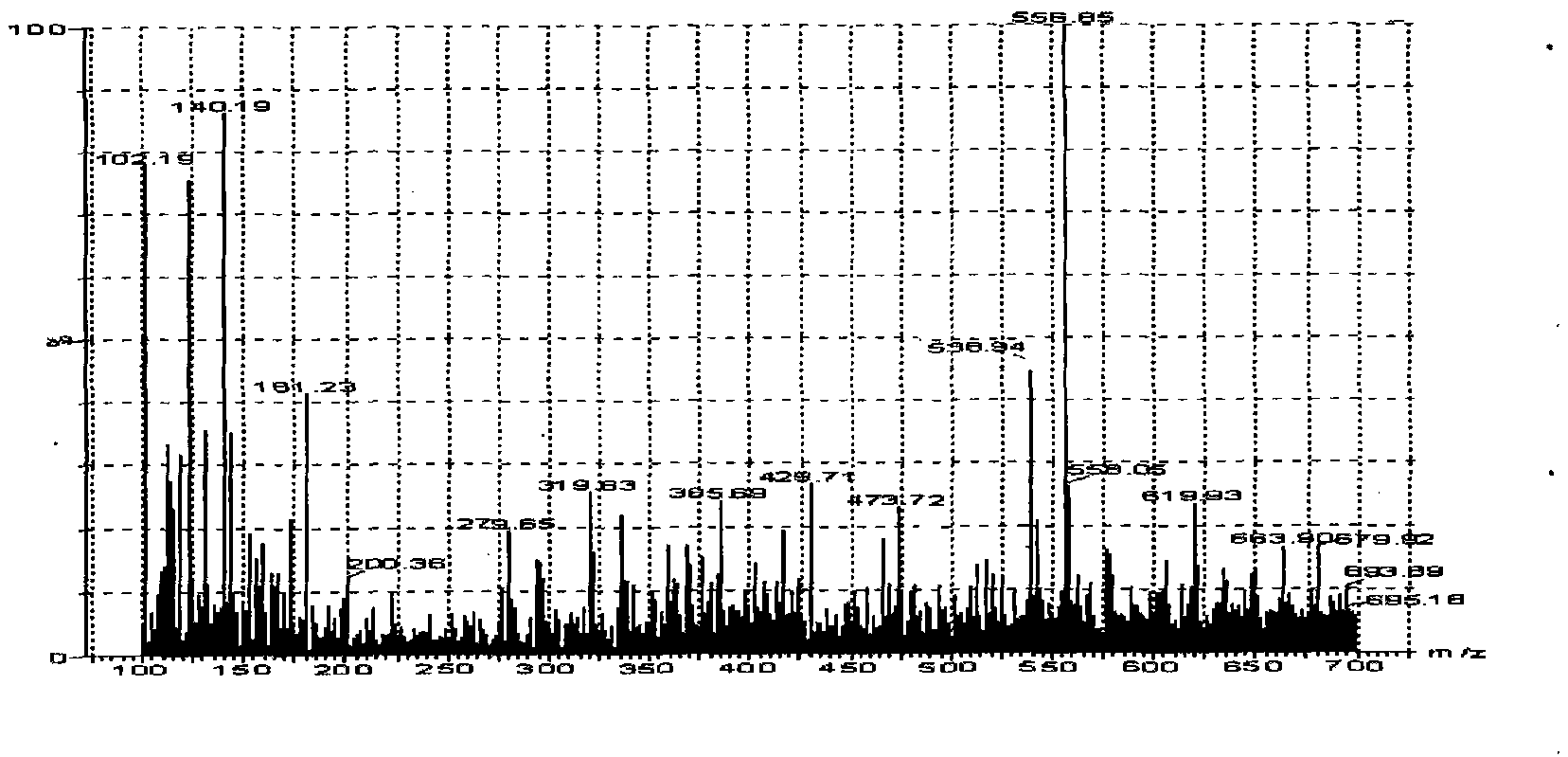
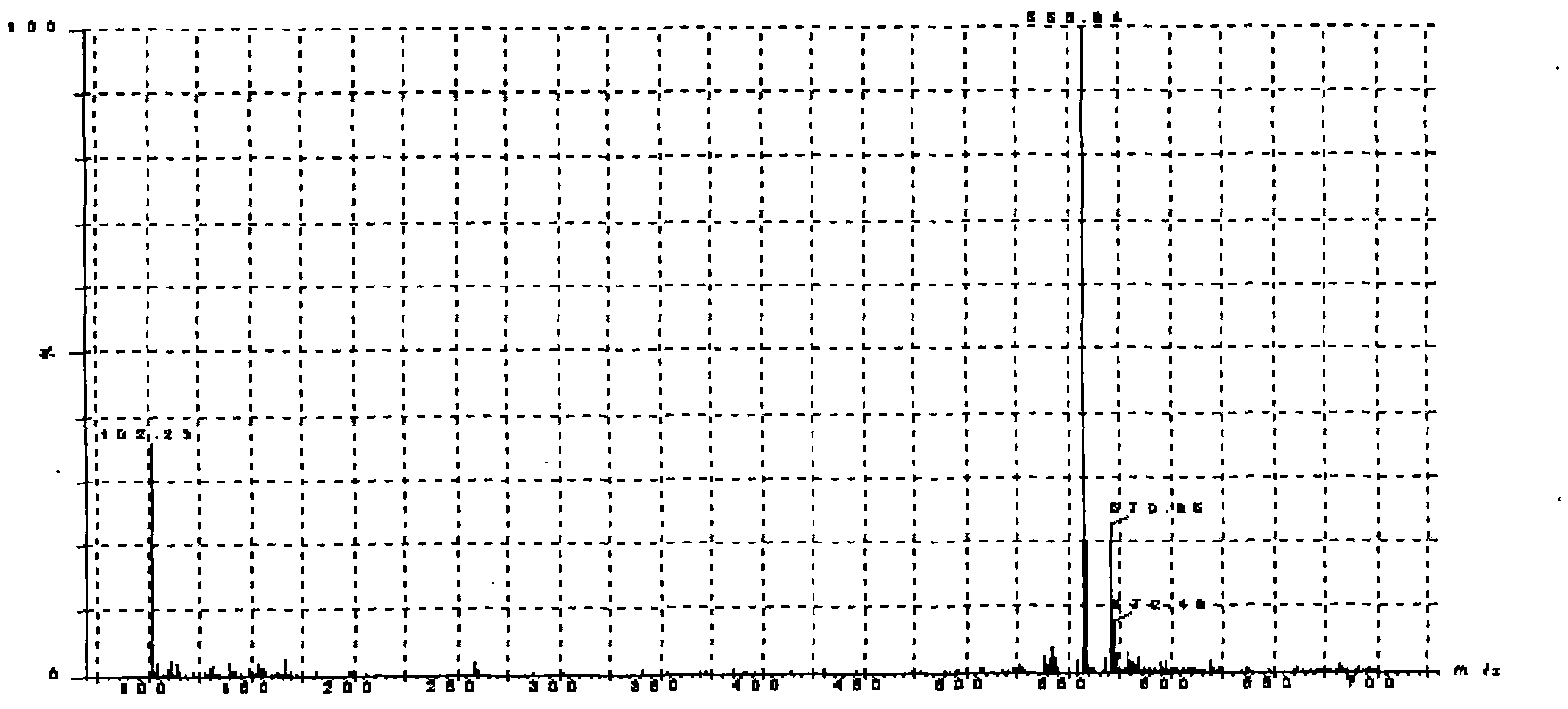
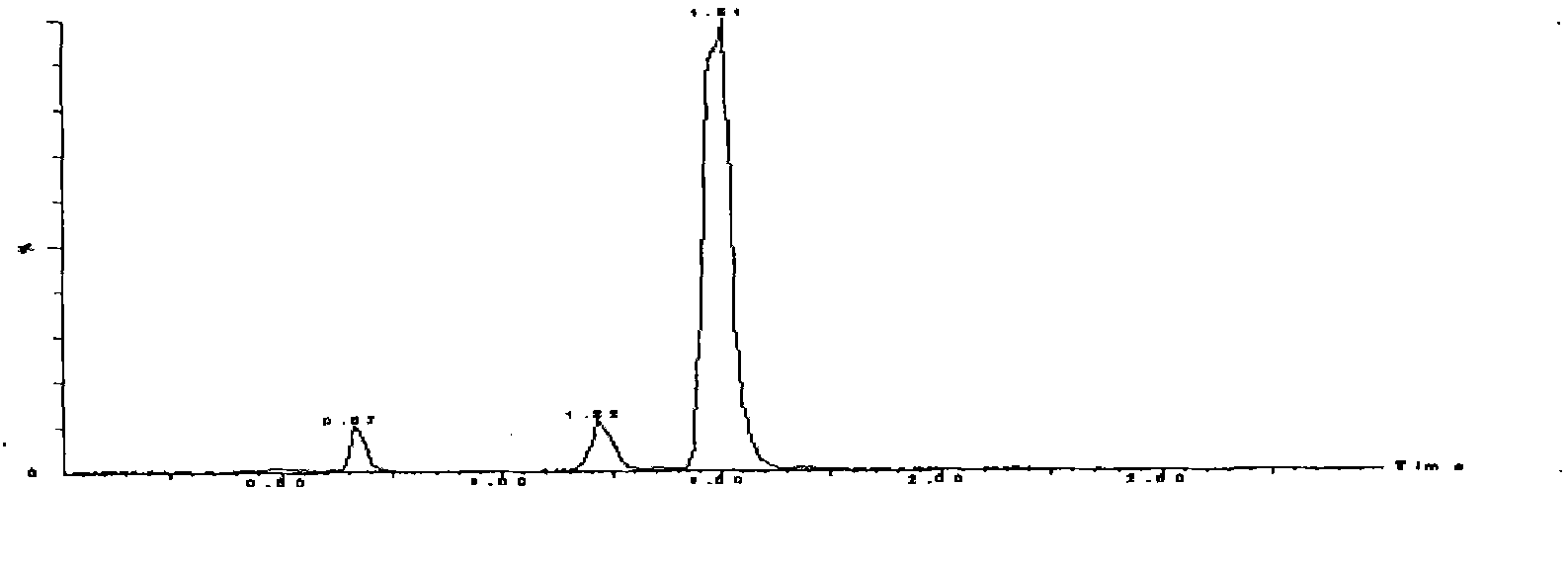
LC-MS/MS method for determining milbemycin oxime content of animal plasma
A technology of milbexime and plasma, which is applied in the field of veterinary drug content determination and pharmacokinetic research, can solve the problems of unfavorable detection, insufficient sensitivity, time-consuming, etc., and achieve high precision and accuracy, small matrix effect, and quantitative low limit effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1 Establishment of LC-MS / MS assay method for milbexime content in animal plasma of the present invention
[0026] 1. Preparation of standard solution Weigh 0.5mg of milbexime standard product, place it in a 100mL volumetric flask, dilute to the mark with acetonitrile, and prepare a standard stock solution equivalent to milbexime 5 μg / mL. According to the needs of the experiment, the standard working solution diluted to the required concentration with the mobile phase was used to draw the standard curve. Both the standard stock solution and the standard working solution are stored at -20°C;
[0027] 2. Plasma sample pretreatment Add 4mL acetonitrile and 0.3g sodium chloride to 1mL plasma sample, vortex fully on the micro-mixer for 1min, centrifuge at 3500r / min for 5min, take the supernatant into a conical bottom glass centrifuge tube, 50℃ nitrogen Blow dry under flow, add 3mL of methanol-5mmol / L ammonium acetate to the residue at a ratio of 1:9, vortex and mi...
Embodiment 2
[0058] Example 2 Practical application of the LC-MS / MS determination method of milbexime content in animal plasma of the present invention and the sample pretreatment method in the study of canine plasma pharmacokinetics
[0059] Milbexime Tablets and healthy Beagle dogs without any acute and chronic diseases were used for method application investigation. Milbexoxime Tablets are Beimaixin, specification 2.5mg / tablet, batch number 090601, produced by Japan Sankyo Pharmaceutical. According to the dose of milbexime 0.25mg / kg, 6 Beagle dogs were given a single oral administration, fasted for 12h before administration, and had free drinking water. After administration, 0.5,1,1.5,2,4,6,8,12, At 24, 36, and 48 hours, 5 mL of blood was collected alternately from the veins of the extremities, anticoagulated with heparin, centrifuged at 3500 r / min for 10 minutes, separated from the plasma, and stored at -20°C for testing. Process and measure according to the plasma sample processing me...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com