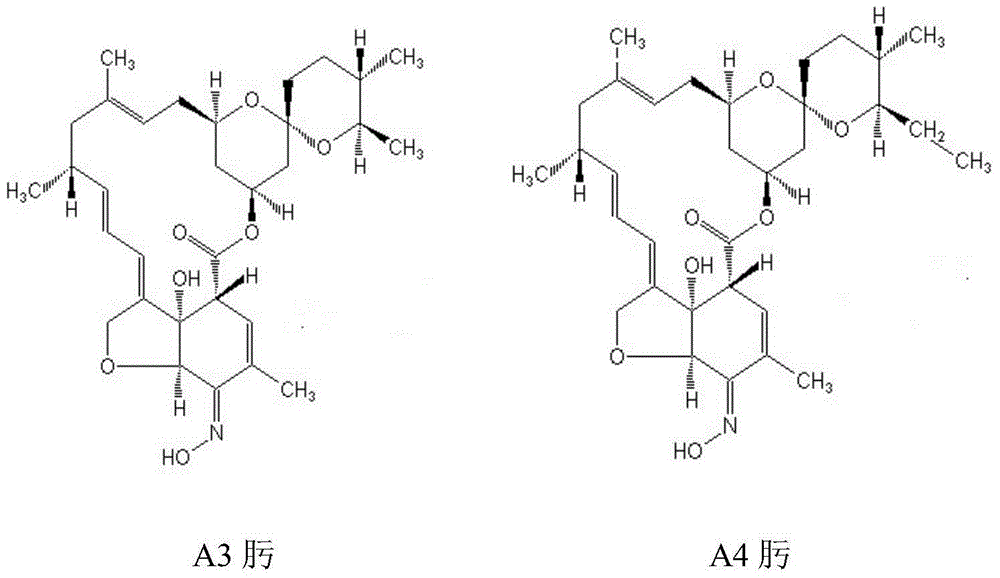
A method of preparing high-purity milbemycin oxime
A Mirbeoxime, high-purity technology, applied in the field of animal pharmacy, can solve the problems of difficulty of mirbeoxime, many components and impurities, difficult separation, etc., and achieves the effects of rapid industrialized large-scale production, low equipment requirements and simple process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Plate-and-frame pressure filtration is performed on the fermentation broth containing milbemycin A3 and milbemycin A4 components, and the slag is extracted with toluene 8 times the weight of the slag, and the obtained toluene extract is detected by high-pressure liquid chromatography to calculate the content of milbemycin Beamycin component 100g. The toluene extract was concentrated until the content of the milbemycin component in the toluene was 15 g / L, and the toluene extract concentrate was filtered through a 0.45 μm pore size sterilizing plate.
[0027] The filtered toluene extract was loaded onto a silica gel column. The silica gel column is filled with 5kg of dry silica gel, and the silica gel particle size is 100-200 mesh. After the column is loaded, the silica gel column is analyzed with the desorbent of toluene:acetone=95:5 (V / V). The stripping liquids were combined, and the obtained stripping liquid contained 90 g of the milbemycin component. A small part of...
Embodiment 2
[0035] Plate and frame pressure filtration is carried out on the fermentation liquid containing milbemycin A3 and milbemycin A4 components, the bacteria residue is extracted with heptane 10 times the weight of the bacteria residue, and the obtained heptane extract is detected and calculated by high pressure liquid chromatography Contains milbemycin component 120g. The heptane extract was concentrated until the content of the milbemycin component in the heptane was 10 g / L, and the heptane extract concentrate was filtered through a sterilizing plate with a 0.2 μm pore size.
[0036] The filtered heptane extract was loaded onto a silica gel column. The silica gel column is filled with 12kg of dry silica gel, and the silica gel particle size is 300-400 mesh. After the column loading is completed, analyze the silica gel column with the desorbent of heptane: butanone = 80:20 (V / V). The stripping liquids were combined, and the obtained stripping liquid contained 110 g of the milbem...
Embodiment 3
[0044] Plate and frame pressure filtration is carried out on the fermentation liquid containing milbemycin A3 and milbemycin A4 components, and the bacterial residue is extracted with heptane 9 times the weight of the bacterial residue, and the obtained heptane extract is detected and calculated by high pressure liquid chromatography Contains milbemycin component 150g. The heptane extract was concentrated until the content of the milbemycin component in the heptane was 13 g / L, and the heptane extract concentrate was filtered through a sterilizing plate with a 0.2 μm pore size.
[0045] The filtered heptane extract was loaded onto a silica gel column. The silica gel column is filled with 15kg of dry silica gel, and the silica gel particle size is 100-200 mesh. After the column loading is completed, analyze the silica gel column with the desorbent of heptane: acetone = 90:10 (V / V). The stripping liquids were combined, and the obtained stripping liquid contained 128 g of the mi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com