Synthetic process of acipimox
A synthesis process and a technology for methylpyrazine, applied in the field of synthesis technology of acyclolimus, can solve the problems of high impurity content of crude product, reduced production efficiency, unstable process, etc., achieves reduction of production cost, simplified production process, reduced The effect of the number of crystals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
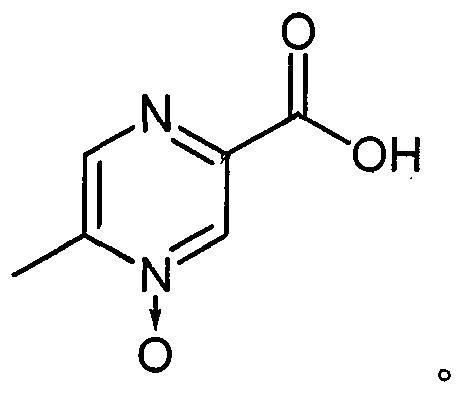
[0027] In the process of studying the preparation process of acipimox, the present inventors discovered unexpectedly through a large number of screening tests: after the reaction mother liquor obtained in the existing acipimox synthesis process is processed by some specific auxiliary agents, Then add activated carbon for decolorization treatment, and a qualified finished product can be obtained through one cooling crystallization. The above process can effectively reduce the number of crystallization times and avoid recrystallization operations, thus greatly simplifying the production process, improving the synthesis yield, and reducing the overall cost.
[0028] Specifically, the present invention provides:
[0029] A synthesis process of acipimox, which comprises:
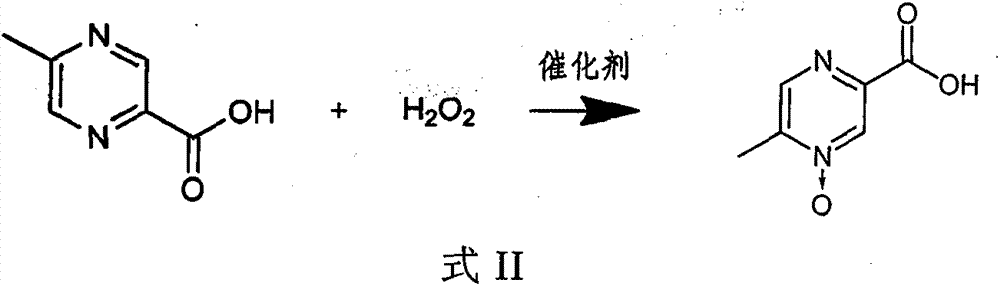
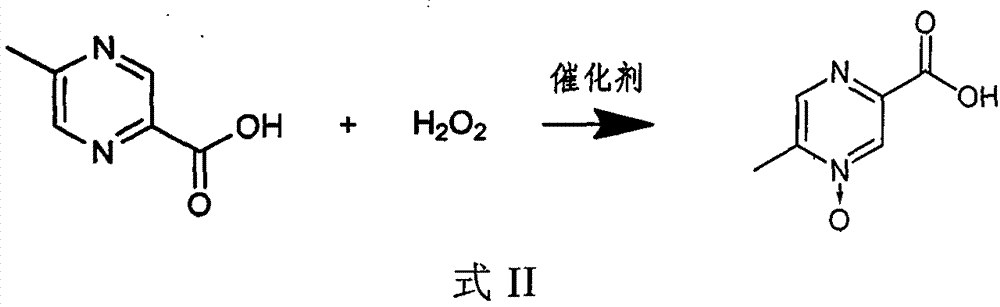
[0030] 1) In an aqueous solution, under the action of a catalyst, 5-methylpyrazine-2-carboxylic acid and hydrogen peroxide react as shown in the following formula II,
[0031]
[0032] 2) adding an auxiliary...
Embodiment 1
[0056] Add 6.2g (19mmol) of sodium tungstate dihydrate into a 1L reaction flask, add 400ml of water, stir to dissolve, add 1.9g (19mmol) of concentrated sulfuric acid while stirring, and continue to add 204g (1.8mol) of hydrogen peroxide (30%), Stir evenly, add 207.2g (1.5mol) of 5-methylpyrazine-2-carboxylic acid, heat in a water bath to 60°C, keep stirring for 8 hours, add 31.2g (0.3mol) of sodium bisulfite, and continue stirring for 1 hour. Add 12 g of activated carbon, continue to stir for 1 hour, filter while it is hot, cool the filtrate to 4 ° C, keep for 3 hours, filter, and dry the filter cake at 100 ° C for 3 hours to obtain 196.2 g of off-white crystalline powder with a HPLC purity of 99.35%. The rate is 84.9%.
Embodiment 2
[0058] Add 6.2g (19mmol) of sodium tungstate dihydrate into a 1L reaction flask, add 400ml of water, stir to dissolve, add 1.9g (19mmol) of concentrated sulfuric acid while stirring, and continue to add 306g (2.7mol) of hydrogen peroxide (30%), Stir evenly, add 207.2g (1.5mol) of 5-methylpyrazine-2-carboxylic acid, heat in a water bath to 80°C, keep stirring for 4 hours, add 151.2g (1.2mol) of sodium sulfite, continue stirring for 0.5 hours, and add 20g of activated carbon , continue to stir for 1 hour, filter while hot, cool the filtrate to -4 ° C, keep for 3 hours, filter, and dry the filter cake at 80 ° C for 3 hours to obtain 192.2 g of off-white crystalline powder, HPLC purity 99.1%, yield 83.1 %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com