A kind of abamectin refining method
A technology of abamectin and a purification method, applied in the field of medicine, can solve the problems of large solvent consumption, cumbersome steps, and high product loss rate, and achieve the effects of saving production time and cost, eliminating oxidized impurities, and reducing the number of crystallization times.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
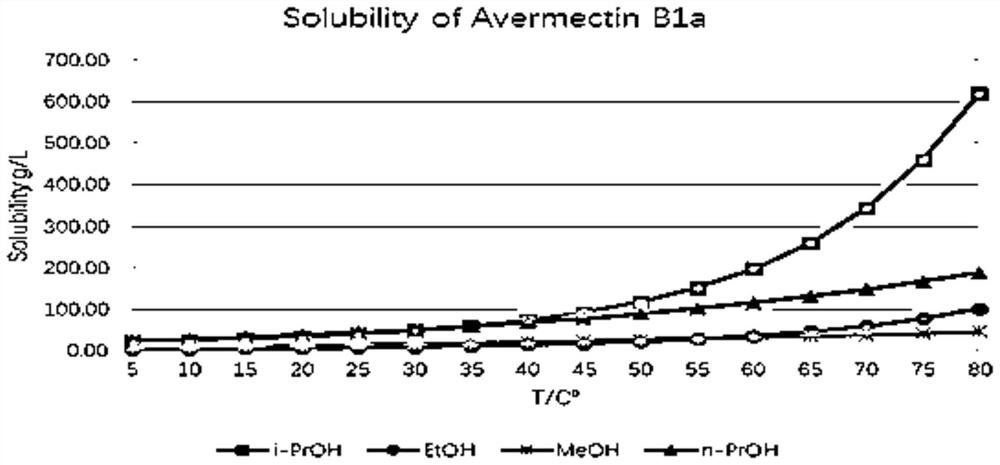
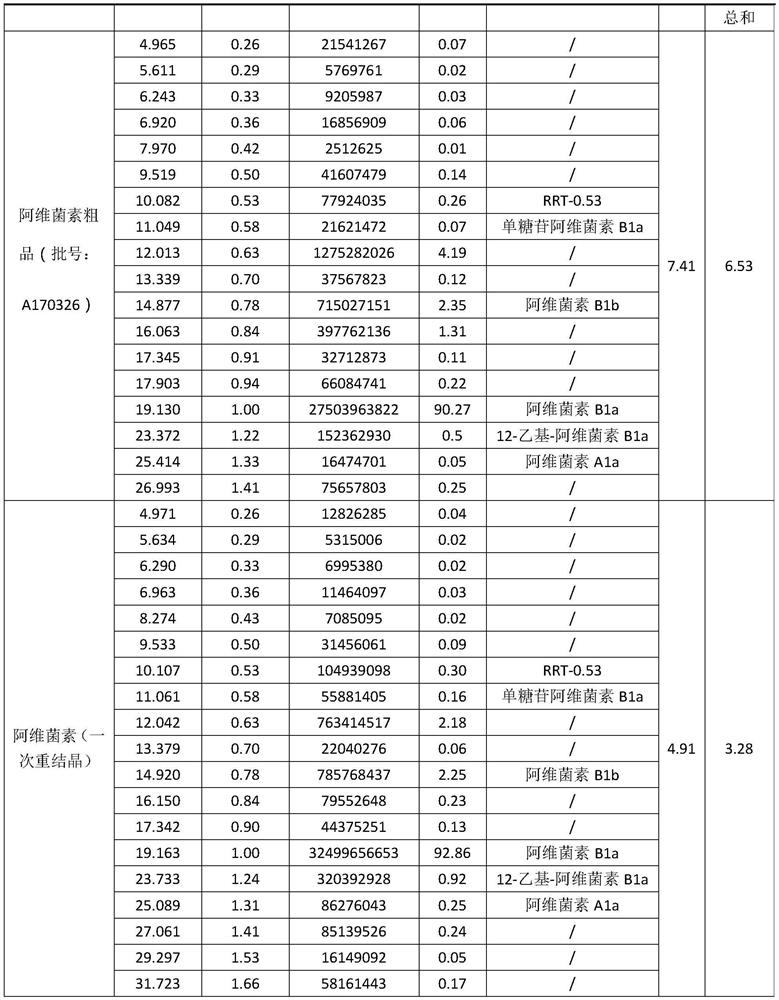
[0041] Embodiment 1, take by weighing 15.0g abamectin crude product (purity 90.27%, oxidized impurity 4.19%) and join in the single-necked bottle of 100ml, in single-necked bottle, add 70ml isopropanol, single-necked bottle is placed in the oil of 85 ℃ Carry out stirring and dissolving in the bath pot to obtain the abamectin crude product solution, after the abamectin crude product solution is carried out the reflux reaction of 2h, slowly drip the formamide of 15ml into the described abamectin crude product solution species, then carry out 5h of Crystallization by cooling, suction filtration of the crude Abamectin solution to obtain a solid, and vacuum drying of the solid at 50° C. to obtain a pure Abamectin product.
[0042] Use high performance liquid chromatography to analyze the crude Abamectin and the pure Abamectin obtained, please refer to Table 1, Abamectin B1a 13.61g, yield 90.73%, purity 92.86%, oxidized impurities 2.18%.
[0043] Table 1
[0044]
[0045]
Embodiment 2
[0046] Embodiment 2, take by weighing the pure Abamectin product among the 8.0g embodiment 1 and join in the single-neck bottle of 100ml as Abamectin crude product (purity 92.86%, oxidation impurity 2.18%), in the single-neck bottle, add 80ml ethanol , the single-necked bottle was placed in an oil bath at 85°C for stirring and dissolving to obtain the abamectin crude product solution, after the abamectin crude product solution was subjected to a reflux reaction for 1.5h, 10ml was slowly added dropwise to the abamectin crude product solution. formamide, followed by cooling and crystallization for 3 hours, suction filtration of the crude Abamectin solution to obtain a solid, and vacuum drying of the solid at 50°C to obtain pure Abamectin.
[0047] The obtained pure abamectin was analyzed by high performance liquid chromatography, please refer to Table 2, and 7.53 g of abamectin B1a was obtained, the yield was 94.13%, the purity was 94.95%, and the oxidized impurity was 0.48%.
...
Embodiment 3
[0050] Embodiment 3, take by weighing 8.8g Abamectin crude product (purity 94.63%, oxidized impurity 0.18%) and join in the single-necked bottle of 100ml, add 70ml ethanol, the single-necked bottle is placed in the oil bath pot of 85 ℃, stirring and dissolving obtains Abamectin crude product solution, after the abamectin crude product solution is subjected to reflux reaction for 1h, slowly add 35ml of formamide to the abamectin crude product solution, carry out cooling crystallization for 3h, to the abamectin crude product solution Suction filtration was performed to obtain a solid, and the solid was vacuum-dried at 50° C. to obtain pure abamectin.
[0051]Use high performance liquid chromatography to analyze the crude Abamectin and the pure Abamectin obtained, please refer to Table 3, obtain Abamectin B1a 8.14g, yield 92.50%, purity 94.92%, oxidized Impurities 0.02%.
[0052] table 3
[0053]
[0054]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com