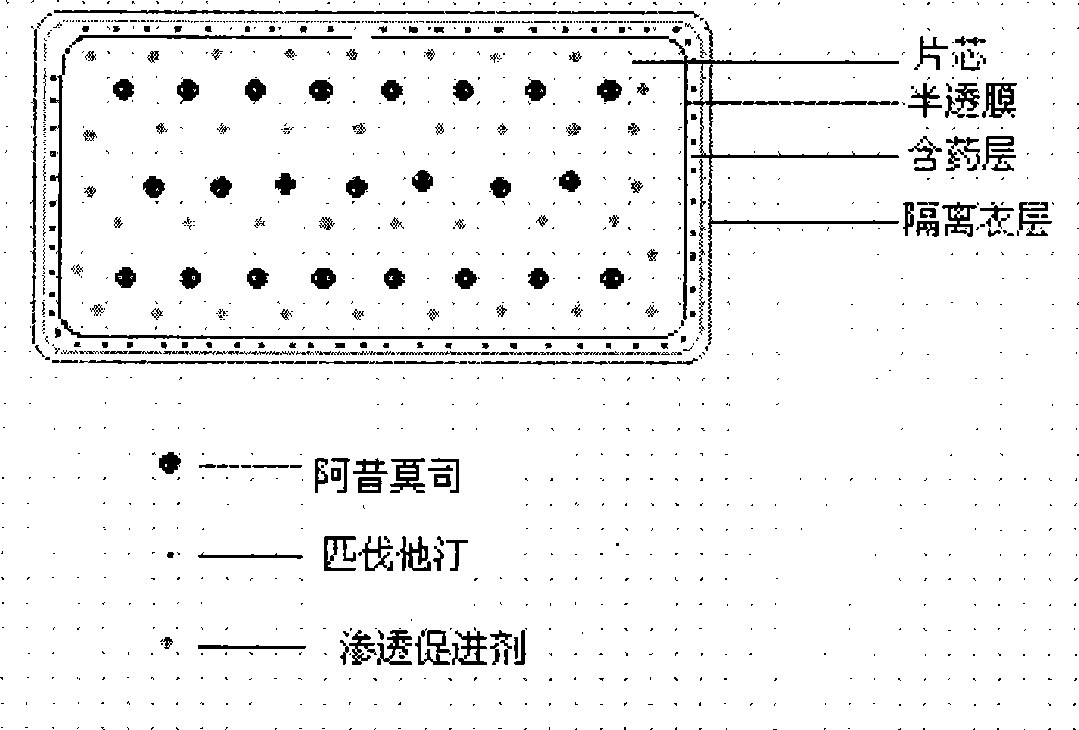
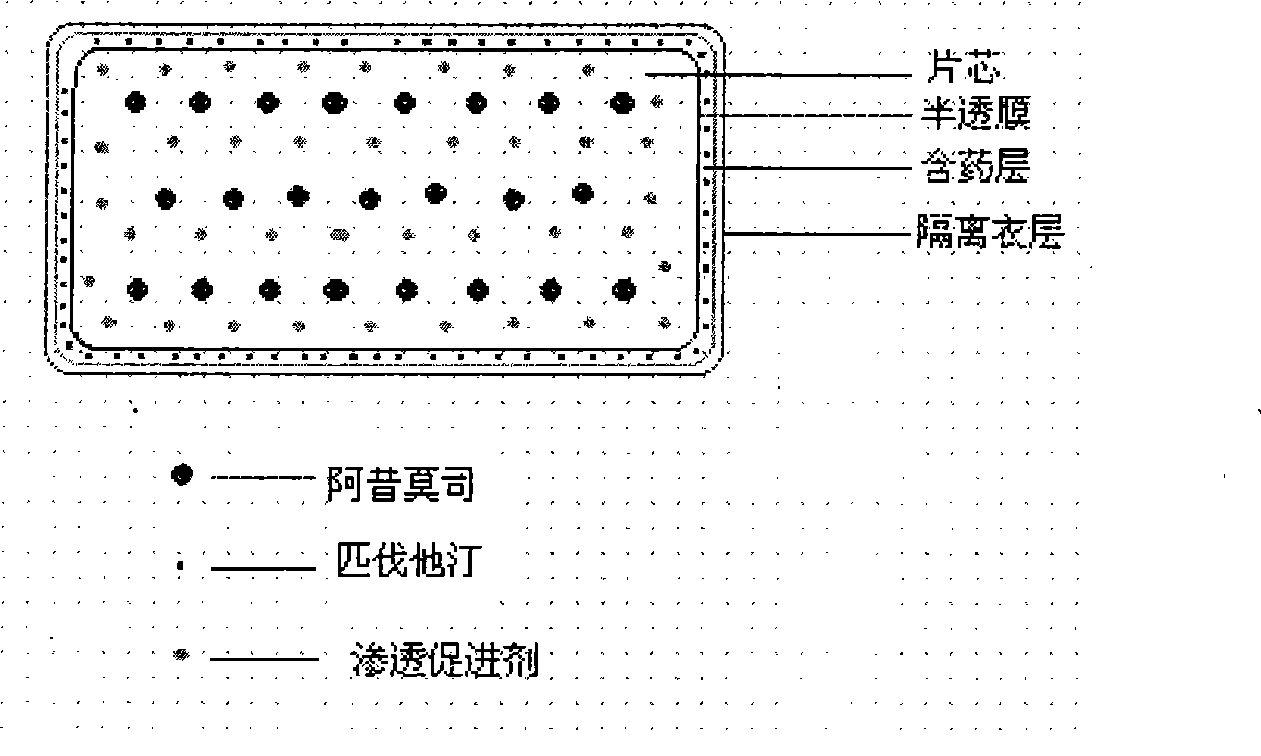
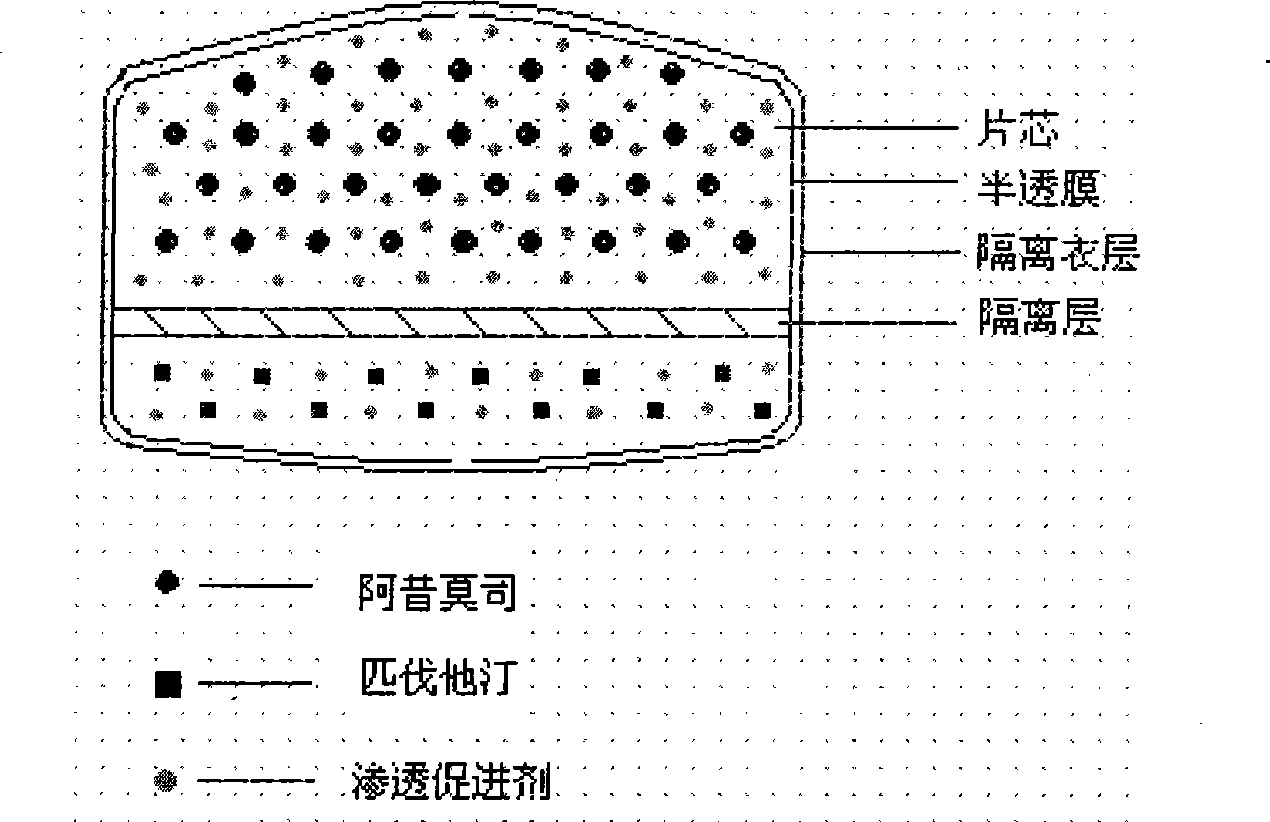
Osmotic pump preparation composition for treating hyperlipemia
A composition and osmotic pump controlled release technology, which is applied in the direction of drug combination, medical preparations with no active ingredients, medical preparations containing active ingredients, etc., can solve the most Optimum ratio and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] Chip composition:
[0086] Acipimus 100g
[0087] NaCl 55g
[0088] PVPk30 2g
[0090] Coating film composition:
[0091] Cellulose acetate 7g
[0092] Macrogol 4000 1.5g
[0093] Diethyl phthalate 1g
[0094] Immediate release drug layer composition:
[0095] Pitavastatin Calcium 1g
[0096] HPMC 6cp 6g
[0098] Isolation film coat layer:
[0099] Opadry II
[0100] Preparation Process:
[0101] (1) Tablet core preparation Take sodium chloride and pulverize, pass through a 100-mesh sieve, mix evenly with acipimox, use 70% ethanol solution containing 8% PVPk30 as a wetting agent, make soft material, pass through a 20-mesh sieve for granulation , dried at 45°C for 2 hours, sized, added with magnesium stearate, mixed evenly, compressed into tablets, about 1000 tablets were compressed using conventional tableting techniques. (2) Tablet core coating: take cellulose acetate, add 280ml of acetone, stir to diss...
Embodiment 2
[0104] Chip composition:
[0105] Acipimus 150g
[0106] Fructose 60g
[0107] Lactose 70g
[0108] PVPk30 5g
[0110] Coating film composition:
[0111] Cellulose acetate 8g
[0112] Macrogol 4000 2g
[0113] Dibutyl sebacate 2g
[0114] Immediate release drug layer composition:
[0115] Pitavastatin Calcium 1g
[0116] HPMC 6cp 6g
[0117] Sodium Lauryl Sulfate 2g
[0118] Titanium dioxide 1g
[0120] Preparation Process:
[0121] (1) Tablet core preparation Take sodium chloride and pulverize, pass through a 100-mesh sieve, mix evenly with acipimox, use 70% ethanol solution containing 8% PVPk30 as a wetting agent, make soft material, pass through a 20-mesh sieve for granulation , dried at 45°C for 2 hours, sized, added with magnesium stearate, mixed evenly, compressed into tablets, about 1000 tablets were compressed using conventional tableting techniques. (2) Tablet core coating: take cellulose acetate,...
Embodiment 3
[0124] Chip composition:
[0125] Acipimus 200g
[0126] NaCl 85g
[0127] pvp k30 5g
[0129] Coating film composition:
[0130] Ethylcellulose 12g
[0131] HPMC6cp 2g
[0132] Macrogol 4000 1g
[0133] Immediate release drug layer composition:
[0134] Pitavastatin Calcium 1g
[0135] HPMC 6cp 6g
[0137] Isolation film coat layer:
[0138] Opadry II
[0139] Preparation Process:
[0140] (1) Tablet core preparation Take sodium chloride and pulverize, pass through a 100-mesh sieve, mix evenly with acipimox, use 50% ethanol solution containing 5% HPMC6cp as a wetting agent, make soft material, pass through a 20-mesh sieve for granulation , dried at 5°C for 2 hours, granulated, added with magnesium stearate, mixed evenly, compressed into tablets, about 1000 tablets were compressed using conventional tableting techniques. (2) Core coating: take ethyl cellulose, add 320ml of ethanol,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com