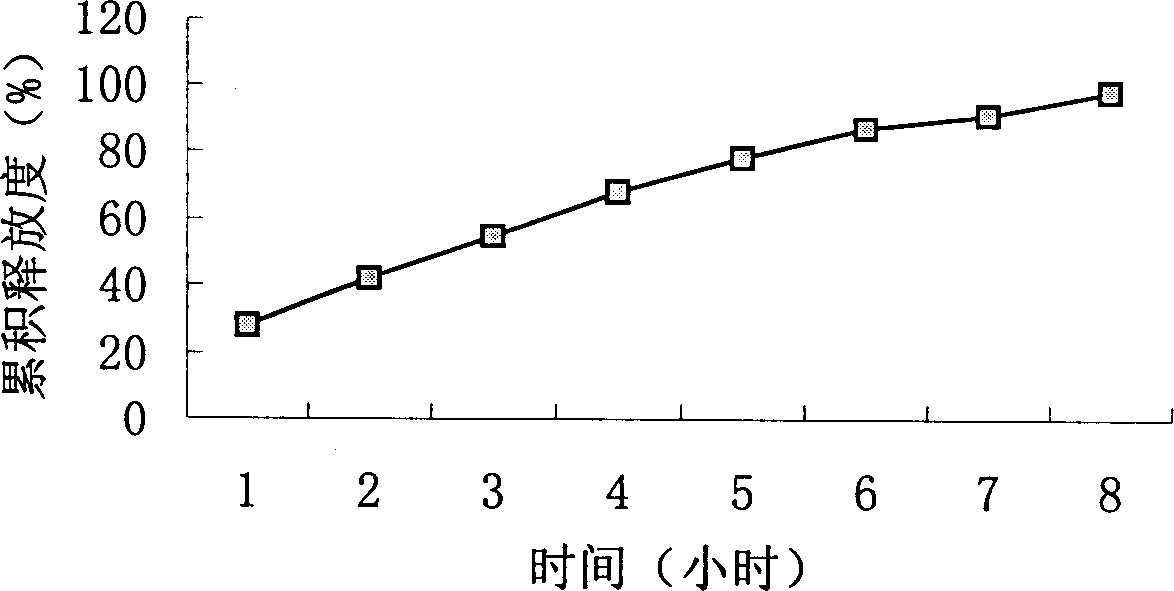
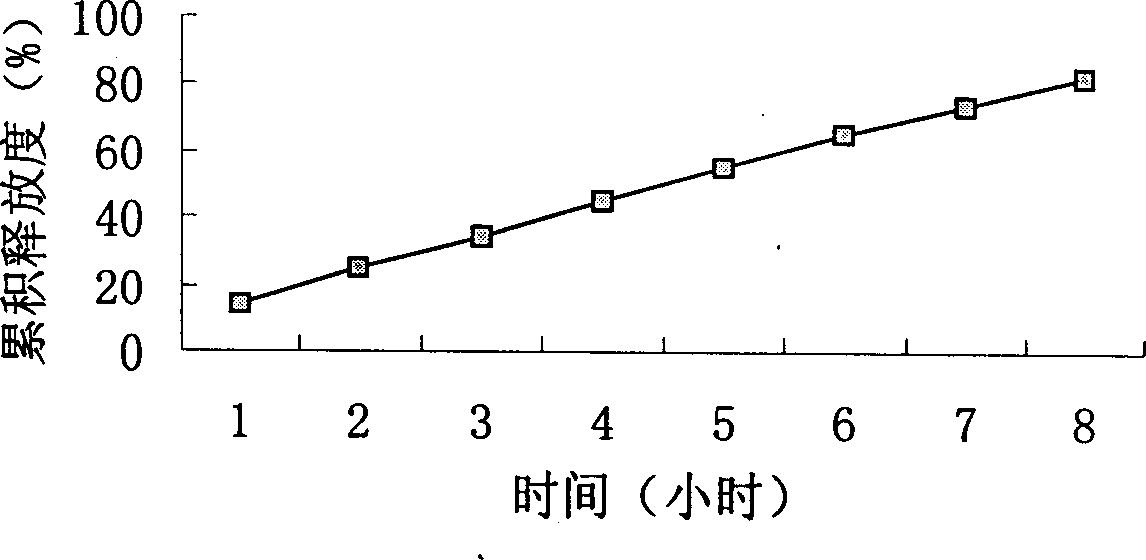
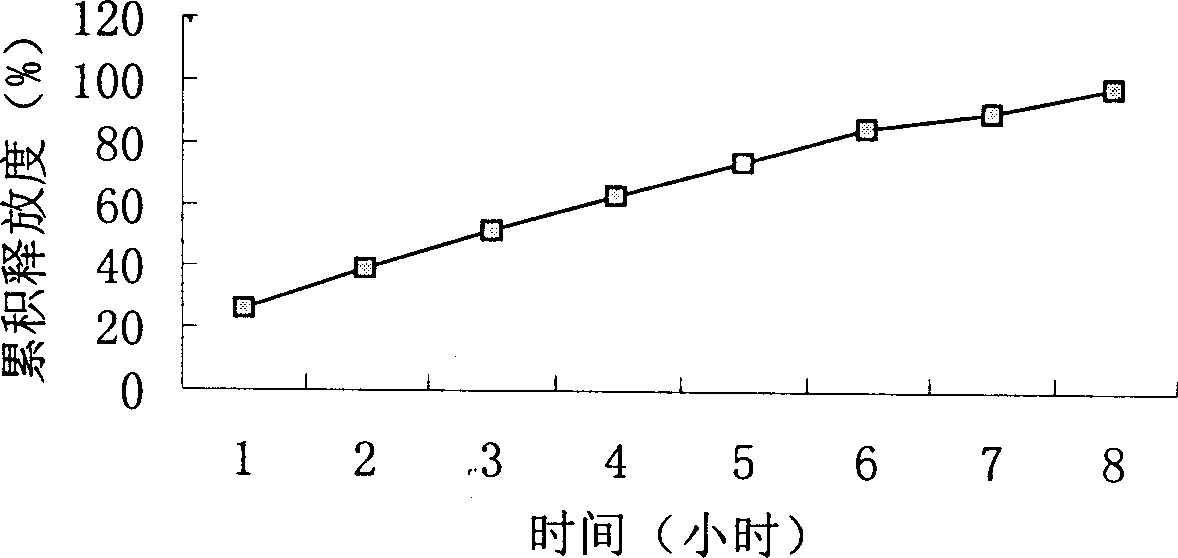
Slow-releasing acipimox tablet
A technology of acipimox and sustained-release tablets, applied in the field of medicine, can solve the problems of diabetes and gout patients with exacerbation of disease, poor safety, and influence on glucose and uric acid metabolism pathways, etc. The effect of benefits and economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0025] Choose from eight specific prescriptions as follows:
[0026] Prescription A: Acipimox 375g
[0027] HPMC-4M 15g
[0028] 8% PVP absolute ethanol solution 150g
[0030] Preparation process: Acipimox passes through a 100-mesh sieve, HPMC-4M passes through a 80-mesh sieve, weighs the prescribed amount of Acipimox and HPMC-4M, mixes them evenly, adds an appropriate amount of 8% PVP absolute ethanol solution, and granulates at 60°C Dried, 16-mesh sieve dry granules, add prescription amount of magnesium stearate to the dry granules, mix well, shape stamping tablets to get final product, about 850 tablets are obtained.
[0031] Prescription B: Acipimox 375g
[0032] HPMC-4M 80g
[0033] 8% PVP absolute ethanol solution 150g
[0035] The preparation process is the same as prescription A.
[0036] Prescription C: Acipimox 375g
[0037] HPMC-15M 15g
[0038] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com