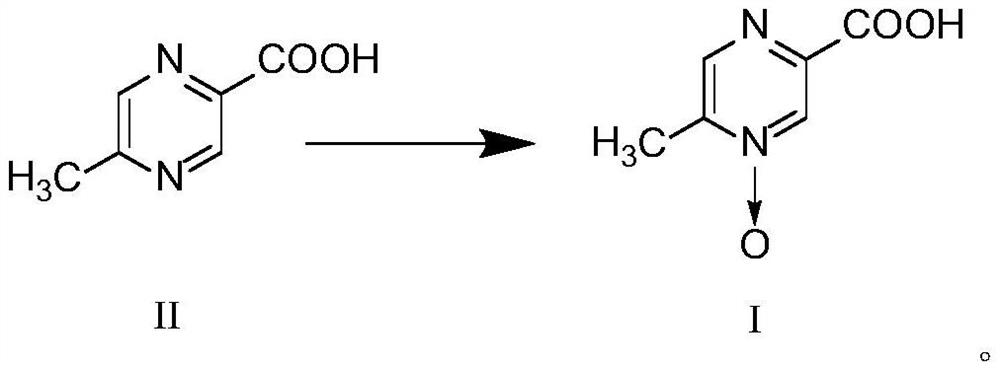
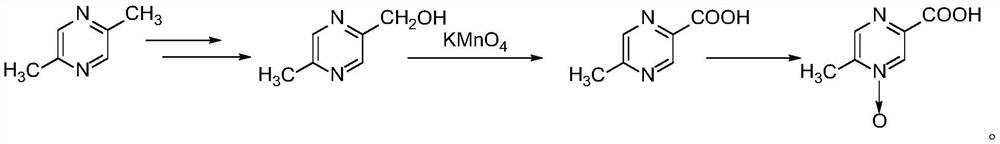
Preparation method of acipimox
A technology of carboxylic acid and methylpyrazine, which is applied in the field of medicine and chemical industry, can solve the problems of high technical requirements, high production cost, and low yield, and achieve the effects of economical and environmentally friendly yield, improved efficiency, and low reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034]Weigh 50.0g of 5-methylpyrazine-2-carboxylic acid, add it to 100.0ml purified water, add 40.0g of concentrated hydrochloric acid, stir for 30min, add 38.5g peroxyacetic acid, stir at room temperature for 3h, use 50% after the reaction is over The pH value of the sodium hydroxide solution was adjusted to 4.0, the temperature of the reaction solution was cooled to 5°C to precipitate crystals of asimimus, which were filtered off with suction and washed with water (20ml), and dried under vacuum at 50°C to obtain asimimus with a yield of 98.5% and a purity of 99.98%.
Embodiment 2
[0036]Weigh 50.0g of 5-methylpyrazine-2-carboxylic acid, add it to 100.0ml purified water, add 30.0g of concentrated hydrochloric acid, stir for 30min, add 38.5g of peracetic acid, stir at room temperature for 2h, and use 50% hydrogen for the end of the reaction The potassium oxide solution was adjusted to pH 4.0, the temperature was lowered to 5°C to precipitate crystals of asimimus, which was filtered with suction, washed with water (20ml), and dried under vacuum at 40°C to obtain asimimus with a yield of 94.5% and a purity of 99.94%.
Embodiment 3
[0038]Weigh 50.0g of 5-methylpyrazine-2-carboxylic acid, add it to 100.0ml purified water, add 25.0g of concentrated hydrochloric acid, stir for 30min, add 31.4g of peroxyformic acid, stir and react at room temperature for 2h, use 50% sodium hydroxide The pH value of the solution was adjusted to 4.0, and the temperature was lowered to 5°C to precipitate crystals of asimimus. After suction filtration, the crystals were washed with water (20ml), and dried under vacuum at 50°C to obtain asimimus with a yield of 92.3% and a purity of 99.89%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com