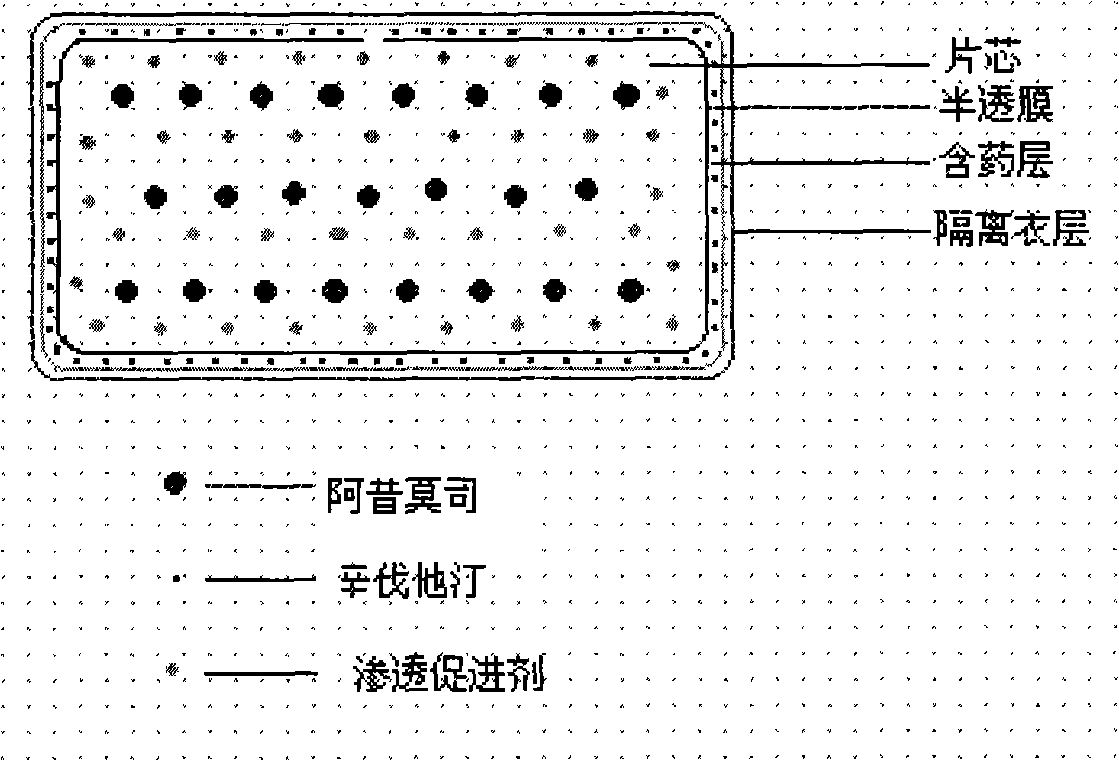
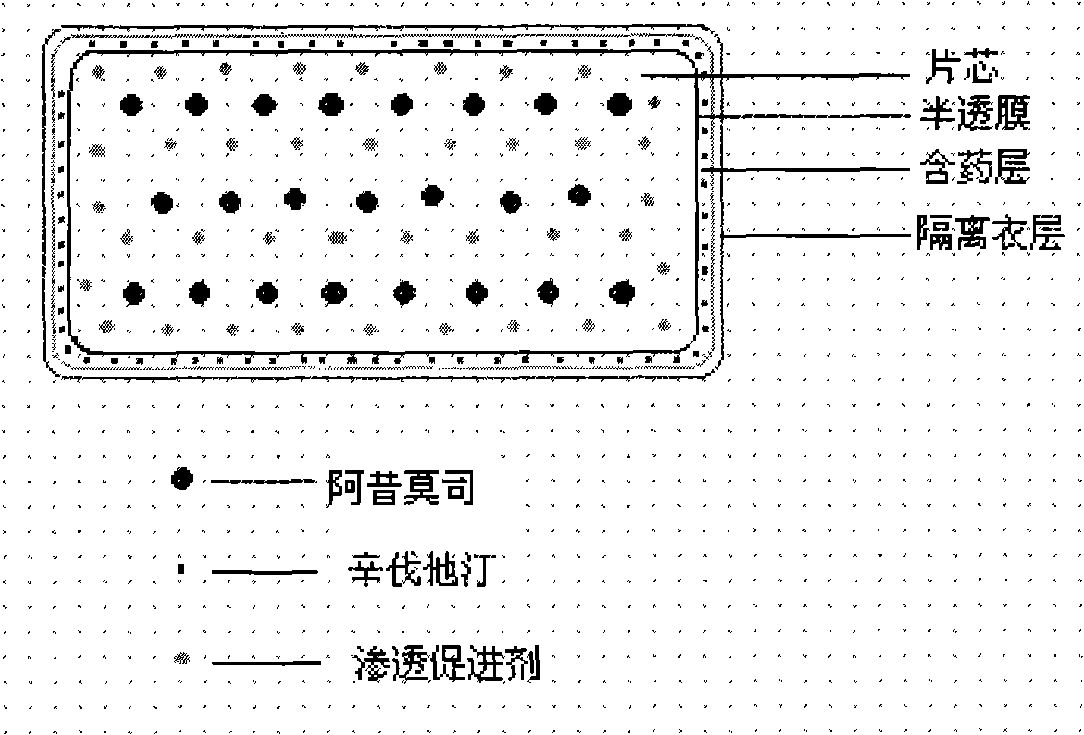
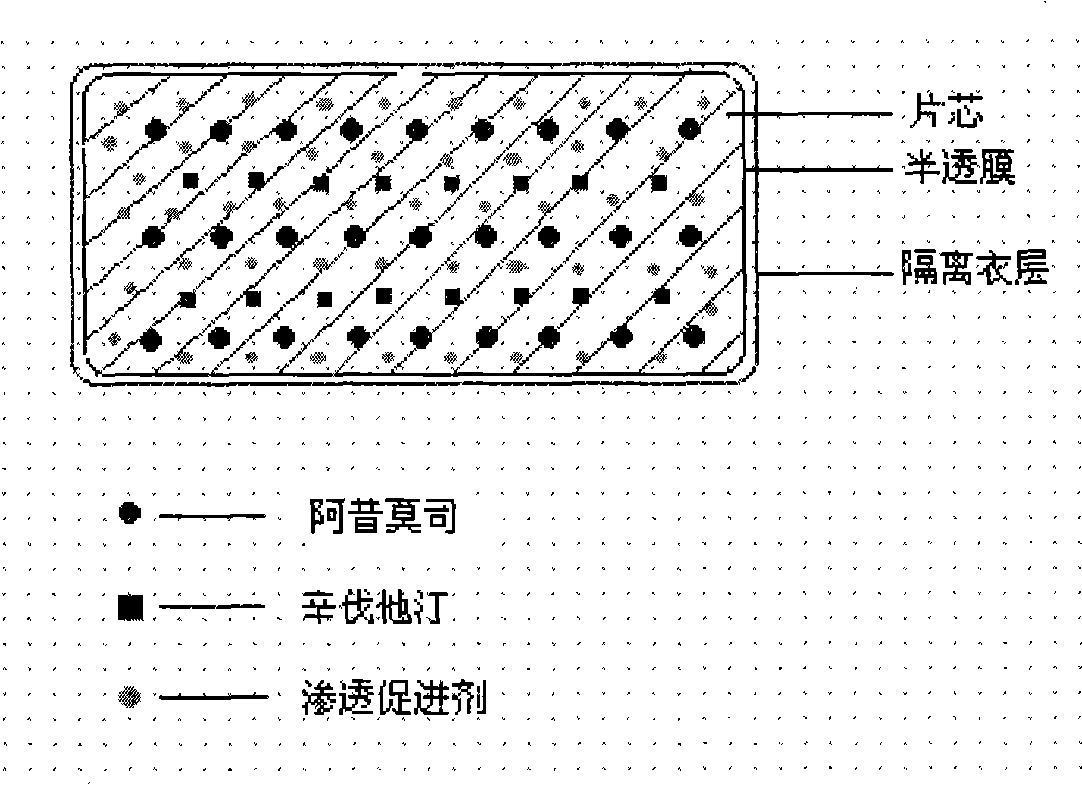
Compound osmotic pump controlled release preparation and preparation method thereof
A technology of controlled-release preparations and osmotic pumps, which is applied in the directions of pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc. than the problem
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0109] Chip composition:
[0110] Acipimus 100g
[0111] NaCl 50g
[0112] PVPk30 2.5g
[0114] Coating film composition:
[0115] Cellulose acetate 6g
[0116] Macrogol 4000 1.5g
[0117] Diethyl phthalate 1g
[0118] Immediate release drug layer composition:
[0119] Simvastatin 10g
[0120] HPMC 6cp 6g
[0122] Isolation film coat layer:
[0123] Opadry II
[0124] Make 1000 tablets by the following preparation method: (1) Tablet core preparation Get sodium chloride and pulverize, cross 100 mesh sieves, mix with acipimox evenly, take 70% ethanol solution containing 8% PVPk30 as wetting agent, prepare Soft material, passed through a 20-mesh sieve to granulate, dried at 45°C for 2 hours, granulated, added with magnesium stearate, mixed evenly, compressed into tablets, and compressed into 1000 tablets by conventional tableting technology. (2) Tablet core coating: take cellulose acetate, add 280ml of acetone, s...
Embodiment 2
[0127] Chip composition:
[0128] Acipimus 150g
[0129] Fructose 45g
[0130] Lactose 45g
[0131] PVPk30 5g
[0133] Coating film composition:
[0134] Cellulose acetate 8g
[0135] Macrogol 4000 2g
[0136] Dibutyl sebacate 2g
[0137] Immediate release drug layer composition:
[0138] Simvastatin 10g
[0139] HPMC 6cp 6g
[0140] Sodium Lauryl Sulfate 2g
[0141] Titanium dioxide 1g
[0143] Make 1000 tablets by the following preparation method: (1) Tablet core preparation Get sodium chloride and pulverize, cross 100 mesh sieves, mix with acipimox evenly, take 70% ethanol solution containing 8% PVPk30 as wetting agent, prepare Soft material, passed through a 20-mesh sieve to granulate, dried at 45°C for 2 hours, granulated, added with magnesium stearate, mixed evenly, compressed into tablets, and compressed into 1000 tablets by conventional tableting technology. (2) Tablet core coating: take cellulose a...
Embodiment 3
[0146] Chip composition:
[0147] Acipimus 200g
[0148] NaCl 90g
[0149] pvp k30 5g
[0151] Coating film composition:
[0152] Ethylcellulose 12g
[0153] HPMC6cp 2g
[0154] Macrogol 4000 1g
[0155] Immediate release drug layer composition:
[0156] Simvastatin 10g
[0157] HPMC 6cp 6g
[0159] Isolation film coat layer:
[0160] Opadry II
[0161]Make 1000 tablets by the following preparation method: (1) tablet core preparation Get sodium chloride and pulverize, cross 100 mesh sieves, mix with acipimox evenly, take the 50% ethanol solution containing 5% HPMC6cp as wetting agent, prepare Soft material, passed through a 20-mesh sieve to granulate, dried at 5°C for 2 hours, granulated, added with magnesium stearate, mixed evenly, compressed into tablets, and compressed into 1000 tablets by conventional tableting technology. (2) Core coating: take ethyl cellulose, add 320ml of ethanol, s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com