STAT3 inhibitor and application in pharmaceutical industry
A technology of inhibitors and drugs, applied in the direction of antipyretics, anti-inflammatory agents, anti-tumor drugs, etc., can solve the problems of no reports and no therapeutic effect of diseases, etc., achieve small toxic and side effects, significant curative effect, anti-inflammatory and anti-cancer The effect of applying the foreground
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
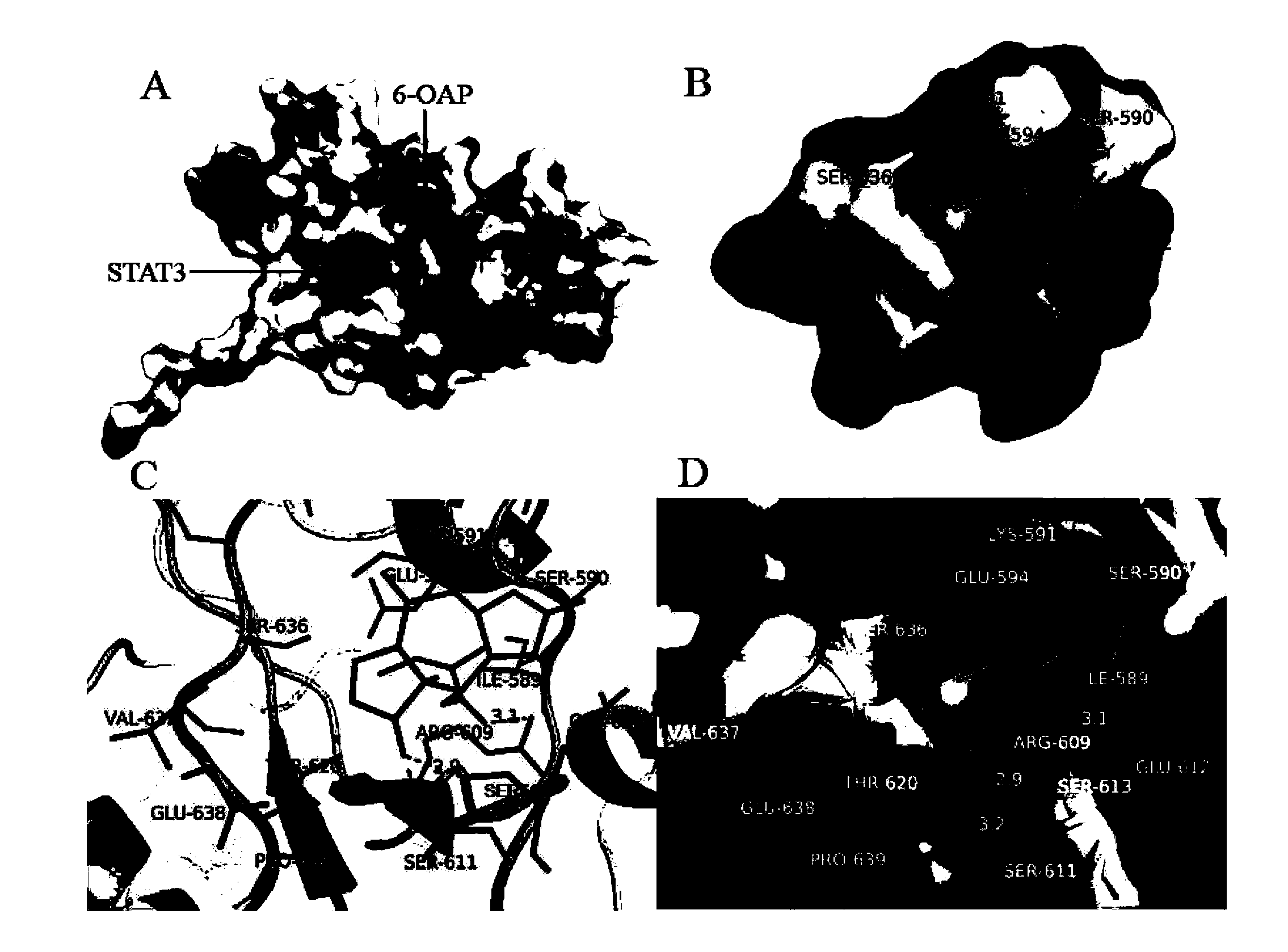
[0043] Use Autodock Vina software to carry out molecular docking on the three-dimensional structure of 6-OAP and STAT3 (refer to the protein database http: / / www.rcsb.org / pdb / ), the SH2 domain of STAT3 protein is used as the receptor, and the 6-OAP and SH2 domains Arg609, ser611 and Ser613 amino acid residues can form hydrogen bonds (see figure 1 B, C, D), the STAT3 and SH2 domains are represented by topological cartoons (see figure 1 C) and electrostatic surface diagram rendering (see figure 1 A, B, D).
Embodiment 2
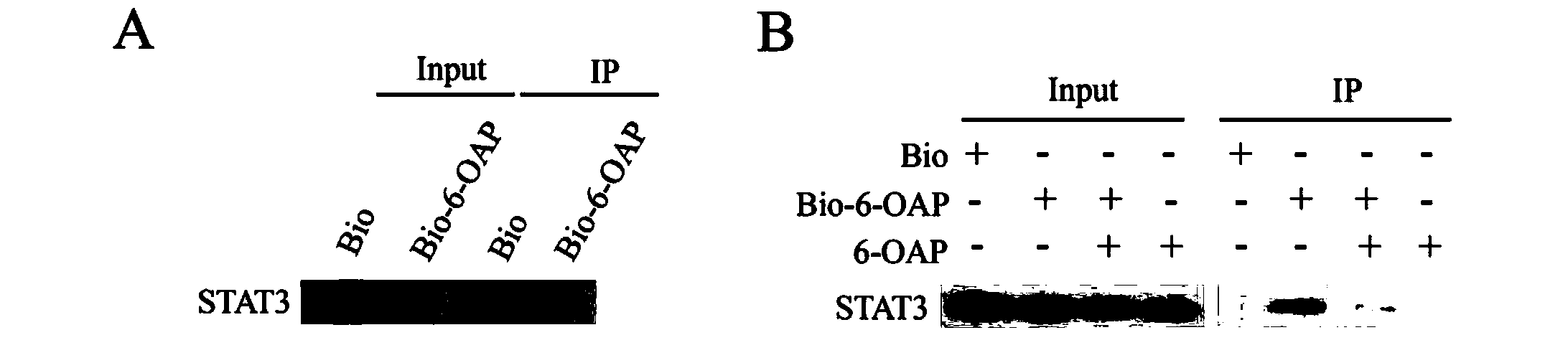
[0045] Treat H1975 cells with 50 μM biotin (Bio) and biotin-labeled 6-OAP (Bio-6-OAP), and lyse the cells after 6 hours. Using the principle of specific binding of avidin and biotin, streptavidin couple The linked agarose beads can precipitate the protein that binds to Bio-6-OAP (Input is the internal control of the total protein amount of STAT3 in the cell, and IP is the STAT3 protein obtained after precipitation), and it is found that Bio-6-OAP can bind to STAT3 (see figure 2 A). In order to further verify the specificity of the binding between 6-OAP and STAT3, H1975 cells were simultaneously treated with 100 μM 6-OAP and 50 μM Bio-6-OAP ("+" means adding the corresponding drug treatment, "-" means adding solvent control) , found that 6-OAP can compete with Bio-6-OAP to bind STAT3, and the amount of Bio-6-OAP bound to STAT3 was significantly reduced (see figure 2 B), indicating that the small molecule compound 6-OAP can specifically and stably bind to STAT3.
Embodiment 3
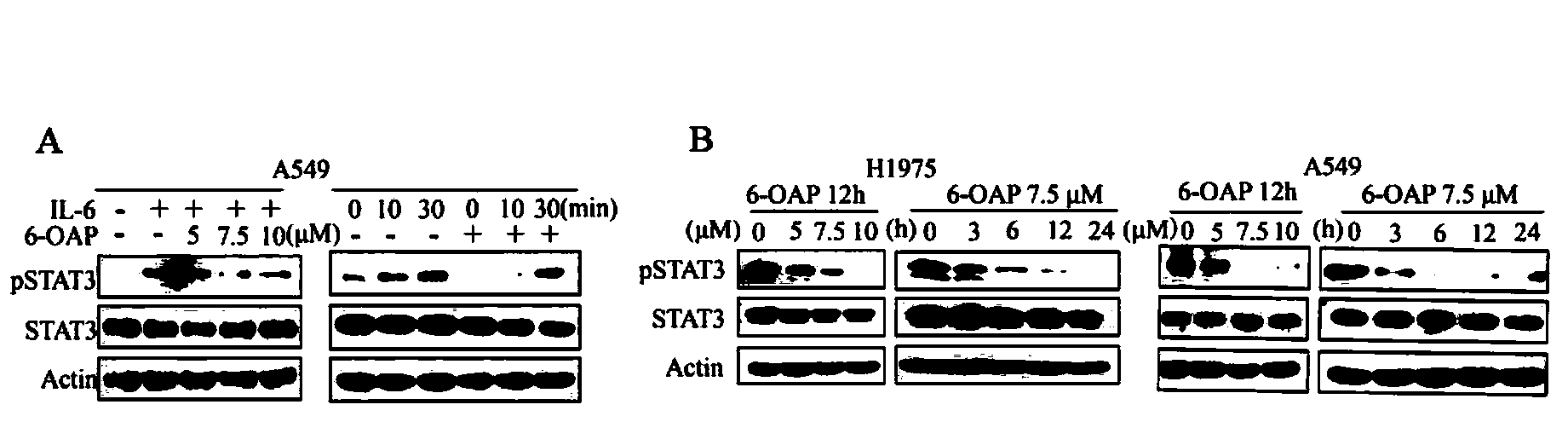
[0047] In order to further detect whether 6-OAP inhibits the activation of Jak2 / STAT3 pathway induced by the inflammatory factor IL-6, A549 cells were starved with serum-free medium first, then incubated with IL-6 (10ng / ml) for 1h, and then treated with different Concentration of 6-OAP (5-10μM) for 3h, or starvation-treated A549 cells were pretreated with 7.5μM 6-OAP for 3h, and then incubated with IL-6 (10ng / ml) for 0-30 minutes before collecting the cells . Experimental detection of the phosphorylation of STAT3 protein found that IL-6 significantly promoted the phosphorylation of STAT3, and after treatment with 6-OAP, this promotion was inhibited (see image 3 A, "+" means adding corresponding drug treatment, "-" means adding solvent control, Actin means total protein internal reference). Also treated H1975 and A549 cells with different concentrations of 6-OAP (5-10μM) for 12 hours (h), or treated H1975 and A549 cells with 7.5μM 6-OAP at different time points (3-24h), found...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com