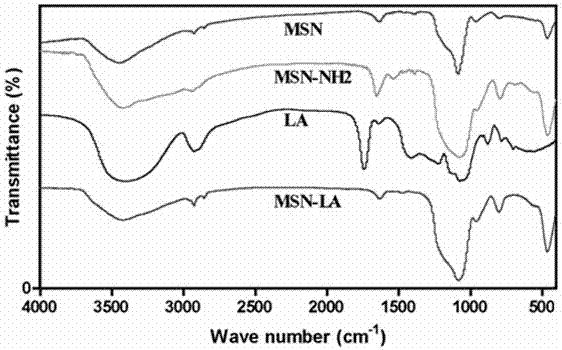
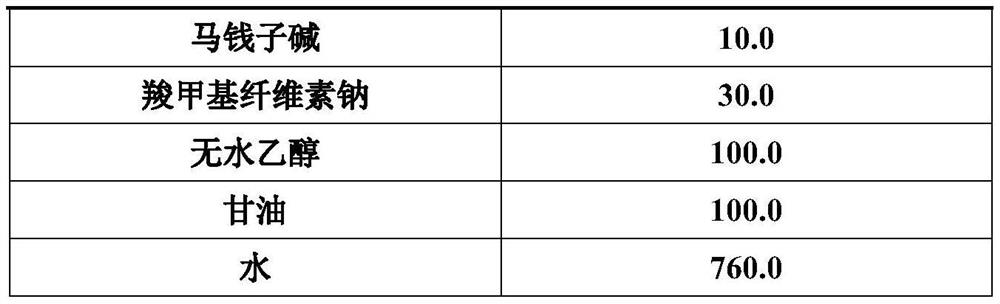
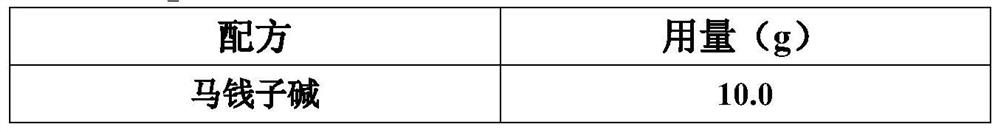
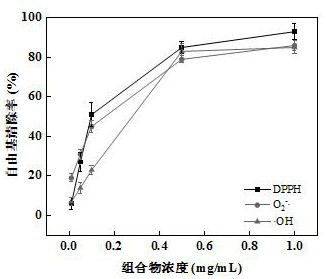
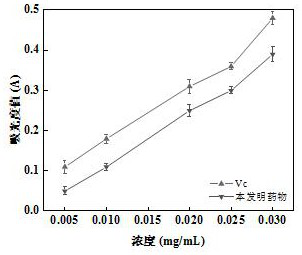
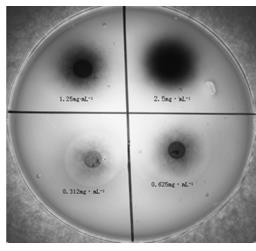
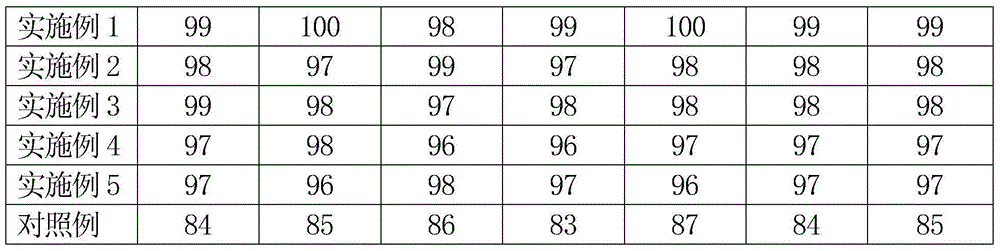
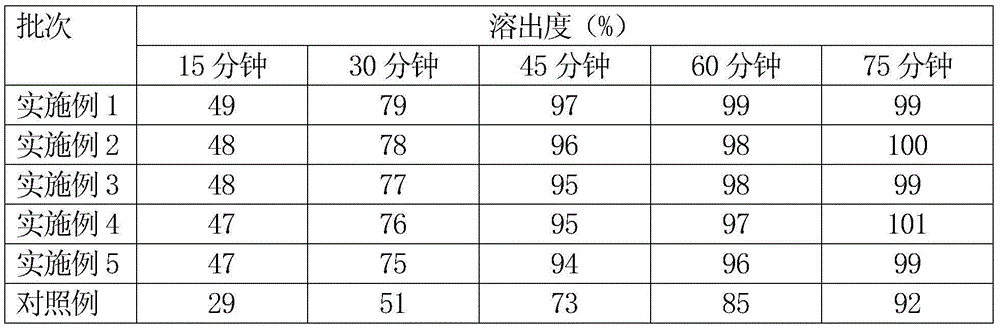
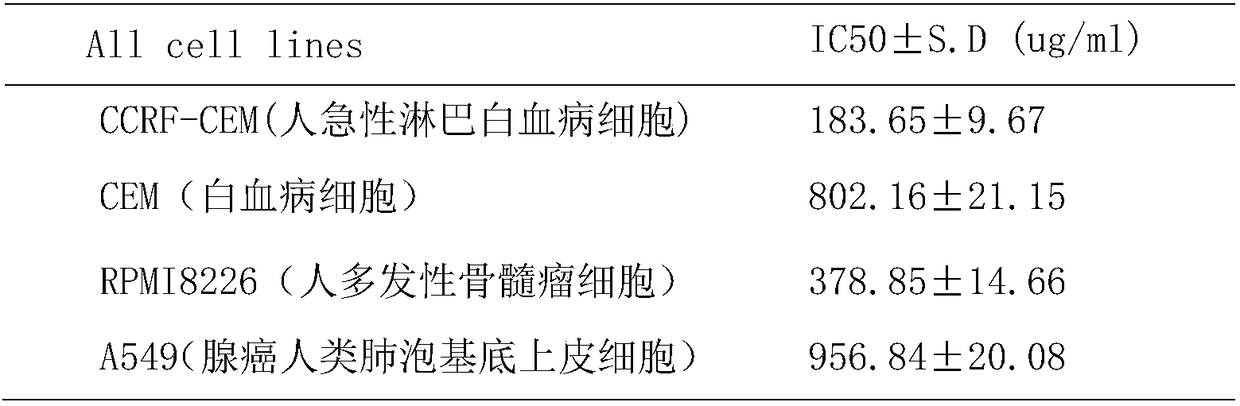
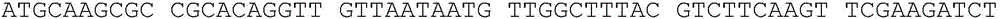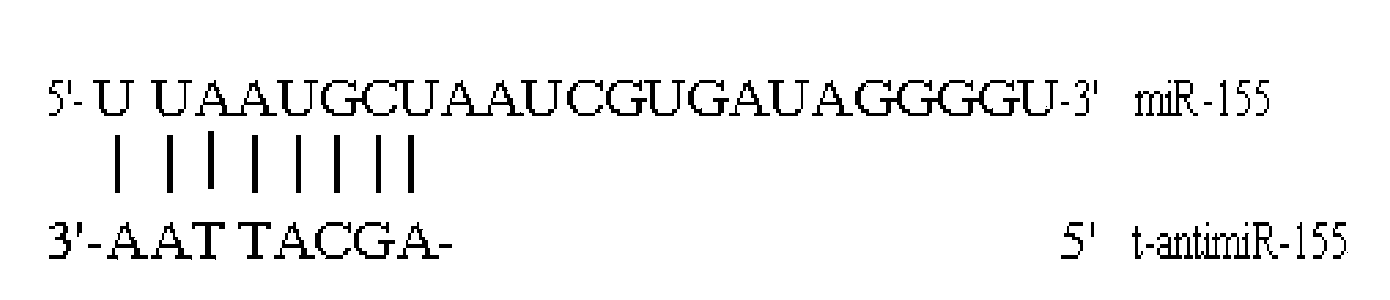Patents
Literature
120results about How to "Toxic and side effects" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
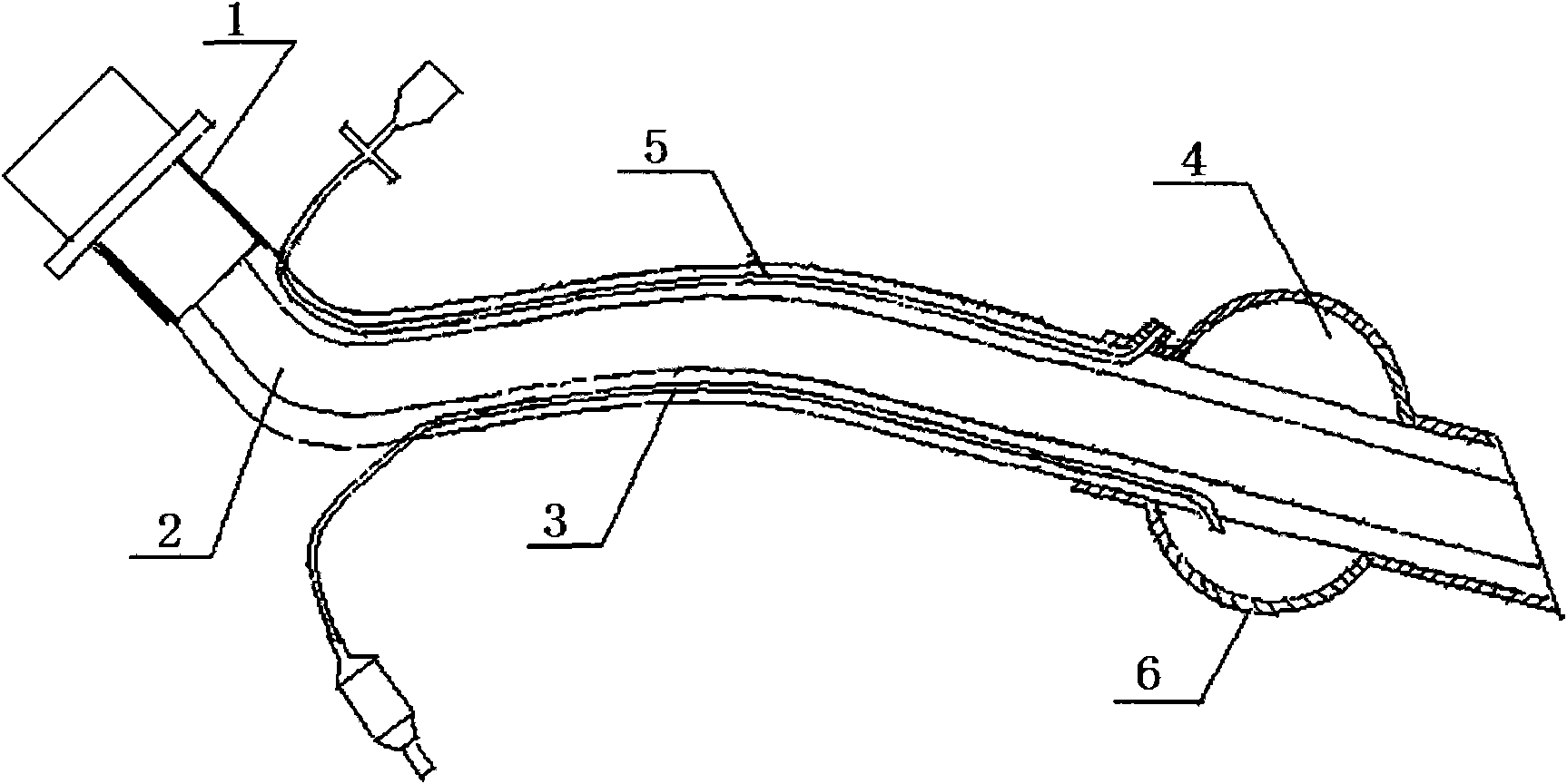
Artificial trachea cannula made of nano materials and preparation method thereof
InactiveCN101579550AAchieve antibacterial effectImprove infectionTracheal tubesCatheterDiseaseRespirator
The invention relates to an artificial trachea cannula made of nano materials in the technical field of medical appliance and a preparation method thereof; the artificial trachea cannula comprises a respirator joint, an air supply tube, an inflation tube, an inflation gasbag, a drain pipe and a lubricant coating, wherein, the inflation tube is in the air supply tube, the output end of the air supply tube is connected with the respirator joint; an inserting section of the air supply tube is cuniform; the external wall of the air supply tube is provided with inflation gasbags; the position where the inflation tube is correspondingly arranged is provided with holes; the drain pipe is embedded on the external wall of the air supply tube and is adjacent to the inflation gasbag; the side of the drain pipe close to the inflation gasbag is provided with a liquid outlet, the other side thereof is connected with an injector; the lubricant coating is arranged on the external wall of the inflation gasbag and the air supply tube, the air supply tube and the inflation gasbag are made of modified plastics. With the method of the invention adopted, damage to lumen tissue when the tube is inserted is reduced, pain is reduced, complicating disease is prevented from occurring; in addition, the preparation process is easy and controllable, the cost is low, as a result, the method is applicable to batch production.
Owner:SHANGHAI NAT ENG RES CENT FORNANOTECH
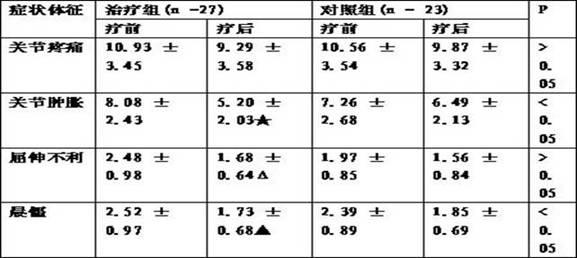
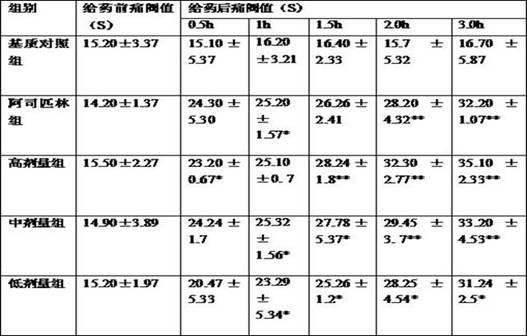
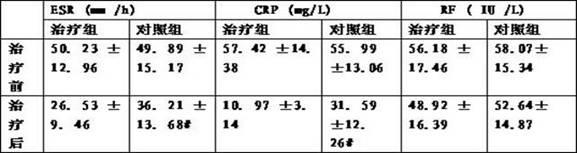
Chinese medicine composition for treating rheumatoid arthritis and preparation method thereof
ActiveCN102178800AEasy to manufactureEffective fastAnthropod material medical ingredientsAntipyreticMedicinal herbsSide effect
The invention discloses a Chinese medicine composition for treating rheumatoid arthritis. An oral-taking medicament is prepared from radix angelicae pubescentis, radix rehmanniae, astragalus mongholicus, angelica, rhizoma drynariae, buthus martensi kirsch, tuckahoe, lumbricus, fructus psoraleae, frankincense, ligusticum wallichii, radix dipsaci, rhizoma alismatis and epimedium; an external use medicament is prepared from rhizoma arisaematis, red-rooted salvia root, radix aconiti kusnezoffii praeparata, chinese ephedra, cassia twig, rhizoma typhonii, frankincense, myrrh, ligusticum wallichii, strychnos, radix angelicae, buthus martensi kirsch, dragon's blood, ground beetle, radix dipsaci and rhizoma acori graminei. The Chinese medicine composition is used for treating rheumatoid arthritis, replenishing vital essence and removing heat and dredging meridians by eliminating dampness and has the advantages of convenience for administration, strong target, easiness for preparation, low cost and capability of reaching affected parts. The Chinese medicine composition is scientifically prepared by utilizing medicinal herbs with different drug properties, can achieve ideal treatment effects, is safe and does not have any toxic or side effect; and through the combination of oral taking and external use, the Chinese medicine composition is capable of replenishing vital essence and removing heat, dredging collateral channels by eliminating dampness and eliminating pains.
Owner:江西博屾医疗器械有限公司
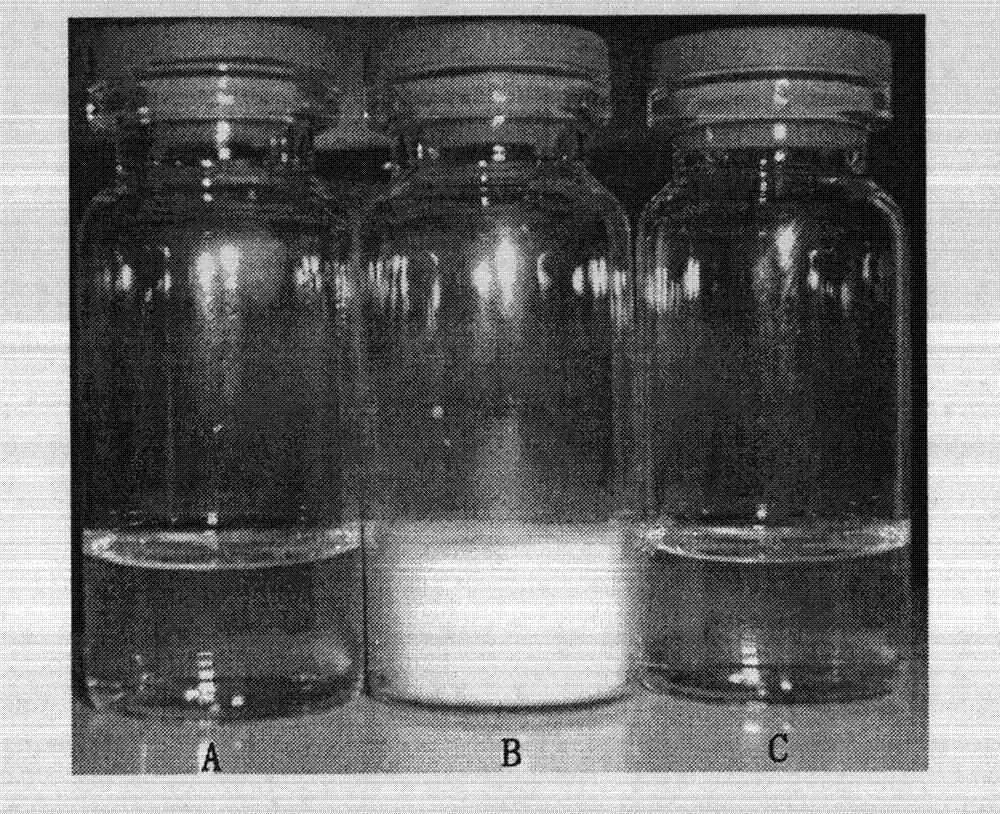
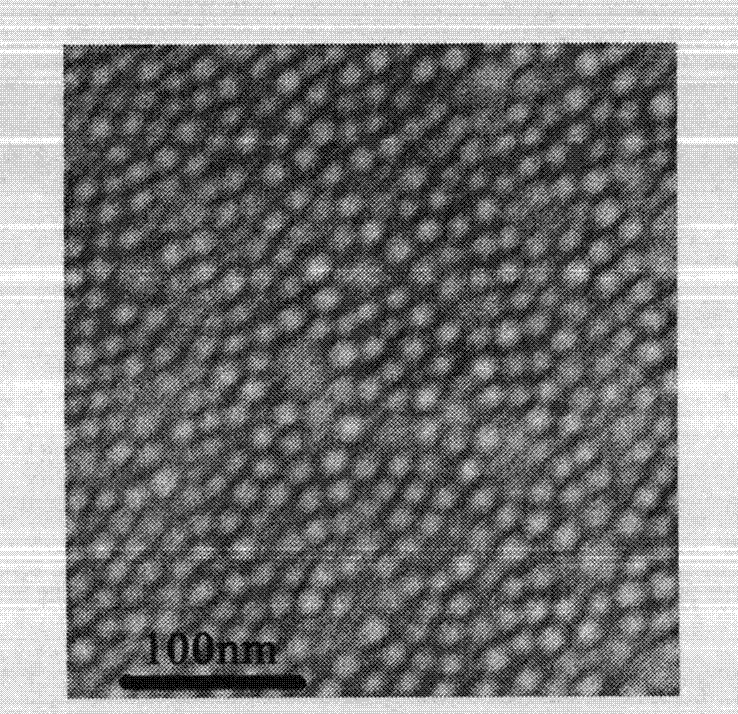
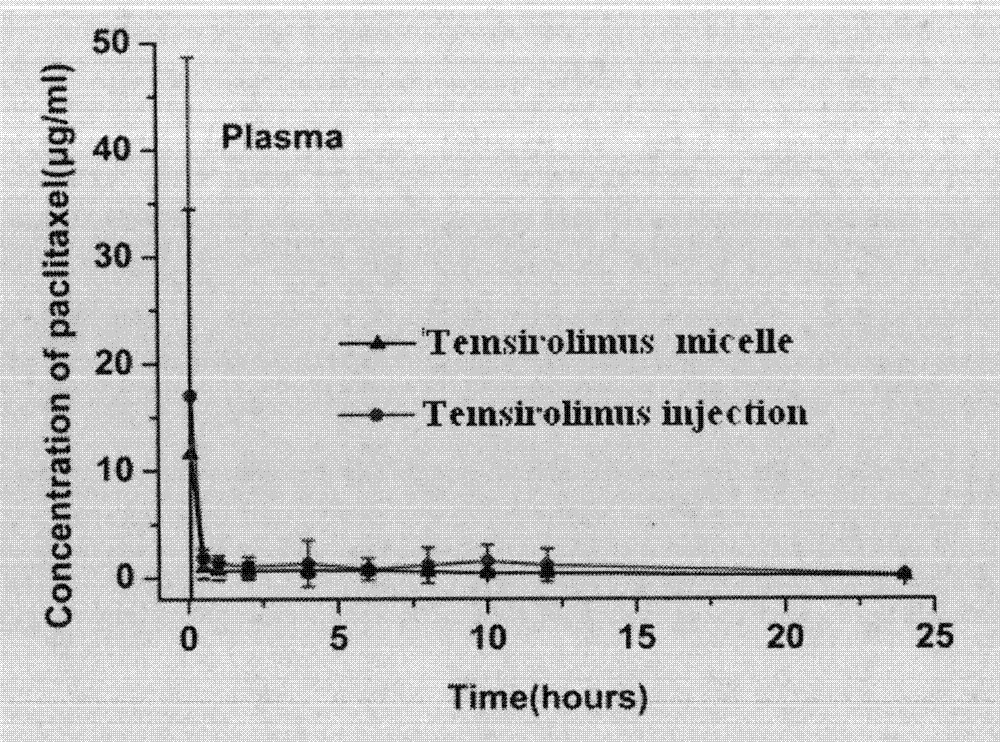
Preparation and application of polymer composition loaded with sirolimus compound or its derivative
InactiveCN103284948AHigh drug loadingHigh encapsulation efficiencyOrganic active ingredientsPowder deliveryPercent Diameter StenosisFreeze dry
The invention relates to a polymer composition loaded with a sirolimus compound or its derivative. The composition can have diversified forms. A necessary link includes preparing the sirolimus compound or its derivative and a polymer carrier into a micelle. Then according to needs, the micelle can be further prepared into a freeze-dried composition by a freeze-drying technology, or the micelle and other polymer and carrier can be prepared into a solid or semi-solid preparation. The polymer composition can be used for treating tumors, reducing rejection reactions after organ and tissue transplantation, promoting cell regeneration and repair, preventing excessive scar tissue growth after injury and preventing vascular restenosis and blood coagulation embolism, treating or inhibiting autoimmune diseases, and treating or inhibiting inflammation, etc.
Owner:单颖
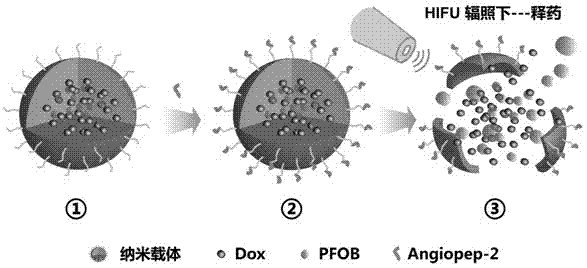
HIFU (high intensity focused ultrasound) controlled-release targeted nanometer drug delivery system of brain glioma, and preparation method and application of targeted nanometer drug delivery system
InactiveCN107050040ASmall particle sizeImprove uniformityUltrasound therapyOrganic active ingredientsSide effectNanocarriers
The invention discloses an HIFU (high intensity focused ultrasound) controlled-release targeted nanometer drug delivery system of brain glioma, and a preparation method and application of the targeted nanometer drug delivery system. The drug delivery system comprises targeting molecules, a drug, a foaming agent and a nano-carrier, wherein the targeting molecules are Angiopep-2 short-peptide, the drug is adriamycin, the foaming agent is perfluorooctane, and the nano-carrier is a high molecular material which is modified by terminal carboxyl and has polylactic acid-glycolic acid copolymer as a main ingredient; the drug and the foaming agent are wrapped and loaded in the nano-carrier together in a wrapping manner; and the targeting molecules are connected to nano-particle surfaces through a covalent linkage manner. The drug delivery system disclosed by the invention can target and highly express the blood brain barrier of an LRP (low-densitylipoprotein receptor-related protein) receptor and brain glioma cells; the accumulation of the drug at a brain glioma position is effectively improved; after the drug is fully accumulated, HIFU irradiation is given to induce the drug to be instantly and fully released at the brain glioma position; the drug concentration in the brain glioma cells is greatly improved; therefore, the treatment effect of the brain glioma is obviously improved; and meanwhile, the toxic and side effect of the drug on normal tissue is effectively reduced.
Owner:EAST CHINA NORMAL UNIV
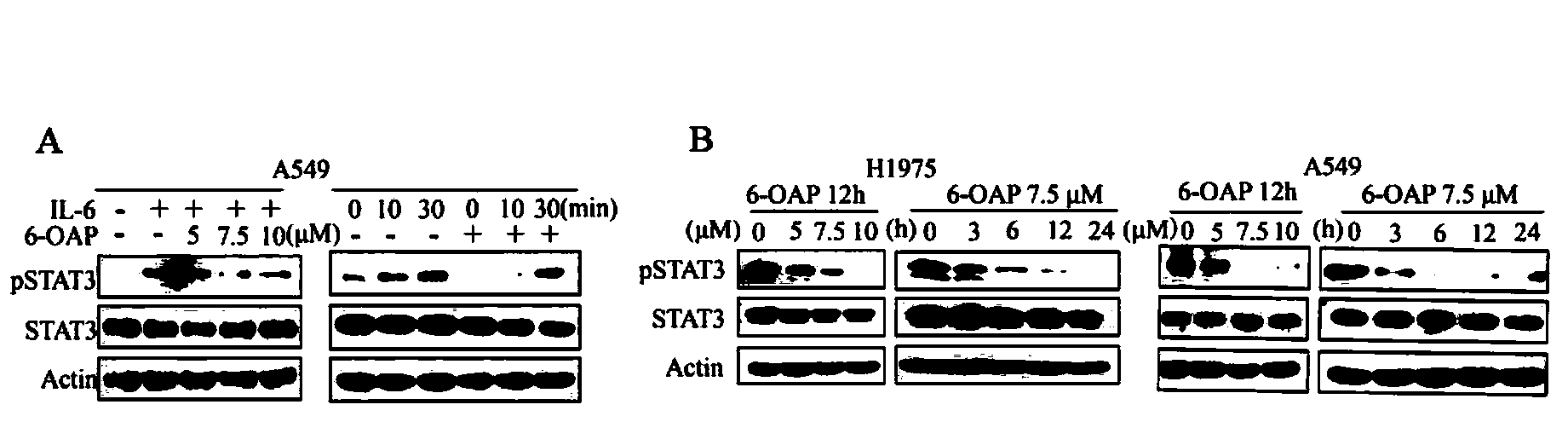
STAT3 inhibitor and application in pharmaceutical industry
InactiveCN104161750AHigh recurrence rateModerate doseOrganic active ingredientsAntipyreticAnti-inflammatorySide effect
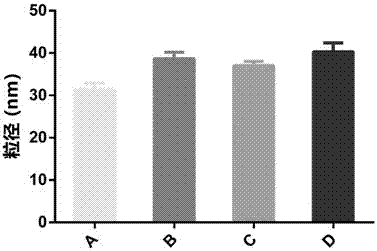
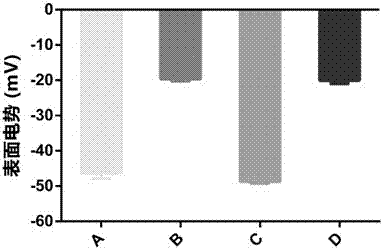
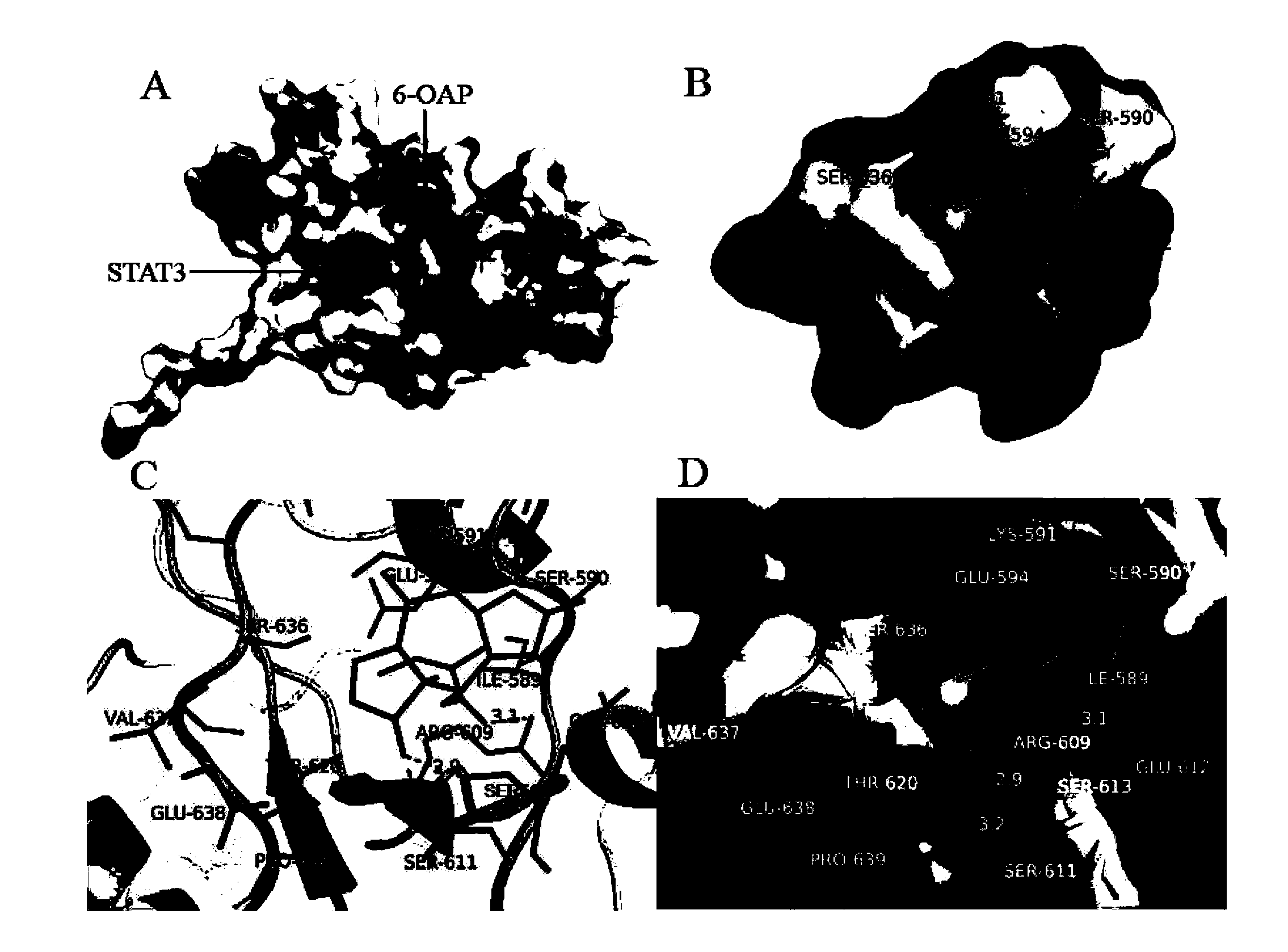
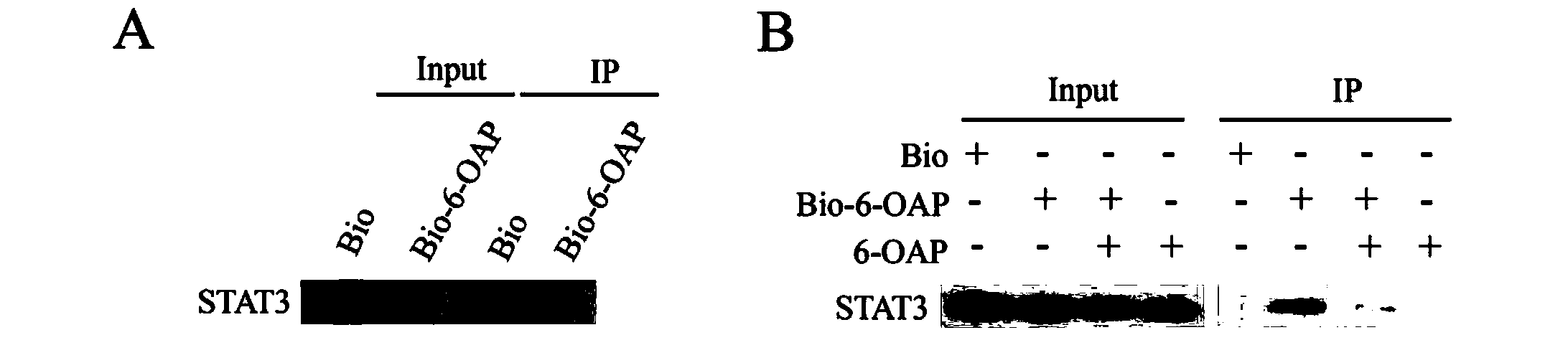
The invention provides application of a sesquiterpene compound 6-OAP in preparation of STAT3 inhibitors and in treatment of diseases unusually relevant to STAT3, and a pharmaceutical composition for treatment of diseases unusually relevant to STAT3. The pharmaceutical composition contains 6-OAP. 6-OAP can quickly combine with STAT3, can significantly inhibit phosphorylation of STAT3 in a cell and animal tumor-bearing model and significantly inhibit the activity of STAT3 induced by inflammatory factors, and has no significant toxic and side effect on other normal tissues. With the advantages of proper dose, significant curative effect, definite action target, and low toxic and side effects, etc., the 6-OAP has wide anticancer and anti-inflammatory application prospects clinically.
Owner:INST OF ZOOLOGY CHINESE ACAD OF SCI +1
Gold paint and preparing process thereof
ActiveCN101033356ARealistic visual effectsNoble metal texturePolyester coatingsCelluloseButyl acetate
The invention relates to a prescription of the gold paint: 50-60% of resin, 4-6% of metal powder or pearl powder, 1-2% of yellow complex dyes, 1-3% of orange complex dyes, 0.5-1.5% of gold powder, 1-2% of UV absorber, 25-33% of diluent, 0.3-0.6% of orientation of metal powder or pearl powder, 0.3-0.6% of anti - sediment agent, 2-3% of n-butyl acetate cellulose. Its preparation includes the following steps: it soaks the diluent and metal powder or pearl powder for 30-50 minutes. And then it slowly disperses the mixture and one of thrird of the resin at a speed of 500-800rpm. And then it adds the pulp which is made from two of third of the resin, orientation of metal powder or pearl powder, anti - sediment agent, gold power through high-speed dispersion, and the pulp which is made from propylene glycol ether acetate and butyl acetate cellulose through high-speed dispersion. And then it adds yellow complex dyes, orange complex dyes, UV-absorber.
Owner:YANCHENG WANCHENG CHEM
Traditional Chinese medicine composition for treating climacteric syndrome and preparation method thereof
InactiveCN103405728AEasy to manufactureLow cost of treatmentInanimate material medical ingredientsSexual disorderSide effectTherapeutic effect
The invention discloses a traditional Chinese medicine composition for treating climacteric syndrome. The traditional Chinese medicine composition is prepared from the following crude drugs: radix bupleuri, radix paeoniae alba, radix curcumae, dogwood, common yam rhizome, mulberry fruit, spina date seed, light wheat, tuber fleeceflower stem, keel, oyster, lotus nut, lily, prepared rehmannia root, rhizoma anemarrhenae, astragalus membranaceus, radix ophiopogonis, schisandra chinensis, fruit of Chinese wolfberry, seed of Chinese dodder, tuckahoe, bighead atractylodes rhizome, root bark of the peony tree, corbicula, soybean sprout, tortoise shell, laportea, bayuezha (Chinese character), filbert, and nacre mother of pearl. The traditional Chinese medicine composition can treat the climacteric syndrome, and can soothe the liver and regulate qi, nourish yin and invigorate the spleen, and nourish yin and yang. The technology has the advantages of being convenient to dose, through in affected part, strong in targeting property, low in cost and the like; and a drug is easily prepared. The traditional Chinese medicine composition adopts different drug properties of traditional Chinese medicinal materials; scientific formulation is carried out; and an ideal treatment effect can be achieved. The traditional Chinese medicine composition is safe, and free of toxic and side effects, can replenish the vital essence and remove heat in combination of oral administration and external application, and can eliminate dampness and dredge channels, and remove pain.
Owner:QINGDAO CENT HOSPITAL
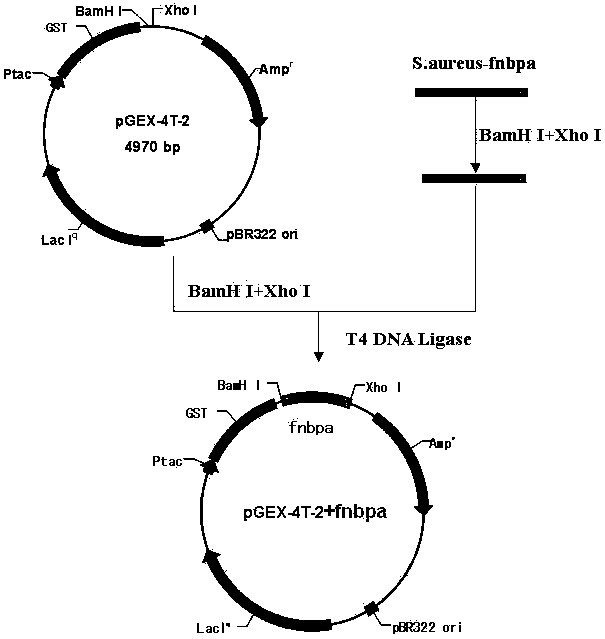
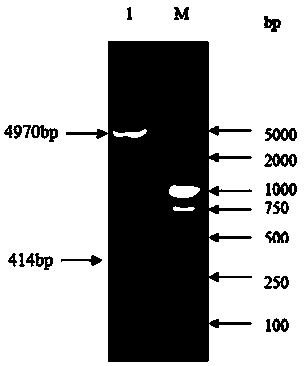
Chemically synthesized staphylococcus aureus surface protein FnBPA gene fragment and expression and application thereof
ActiveCN103725697AHigh titerIncrease the body's cellular immunityImmunoglobulins against bacteriaDepsipeptidesPolyclonal antibodiesGenetic engineering
The invention relates to a chemically synthesized staphylococcus aureus surface protein FnBPA gene fragment, as well as expression and application thereof, belonging to the fields of genetic engineering technologies, antibodies and kits. According to the chemically synthesized staphylococcus aureus surface protein FnBPA gene fragment provided by the invention, a strong antigen epitope, namely 133 amino acids in total from 745th amino acid to 877th amino acid in the staphylococcus aureus surface protein FnBPA are screened out through computer analysis, a codon preferred by prokaryotes is selected, a brand new gene sequence of the antigen epitope is chemically synthesized, the gene fragment is expressed by utilizing the genetic engineering technology, and the strong antigen epitope fragment of the staphylococcus aureus surface protein FnBPA is prepared. The expressed strong antigen epitope fragment of the staphylococcus aureus surface protein FnBPA can be used for detection of staphylococcus aureus antibodies and immune preparation of anti-staphylococcus aureus monoclonal antibodies, polyclonal antibodies and the like.
Owner:李越希
Monoclonal antibody and preparation method thereof
InactiveCN106397581AHigh affinityStrong specificityImmunoglobulinsGenetic engineeringSpleen cellMyeloma cell
The invention discloses a monoclonal antibody and a preparation method thereof. The monoclonal antibody comprises anti-PGAM, CoI alpha, Trx, PGAM 13, CoI alpha 12 and Trx 11. The preparation method comprises the following steps: immunizing mice by embedding PGAM, CoI alpha, Trx, PGAM 13, CoI alpha 2 and Trx 11 in spleens; fusing spleen cells of the mice with myeloma cells SP2 / 0 of the mice; and detecting the cells by indirect ELISA and culturing supernatant. 3, 2, 5, 3, 4 and 3 strains of monoclonal antibodies for resisting PGAM, CoI alpha, Trx, PGAM 13, CoI alpha 12 and Trx 11 are respectively obtained, and homotypes of the antibodies are IgM.
Owner:BINZHOU MEDICAL COLLEGE
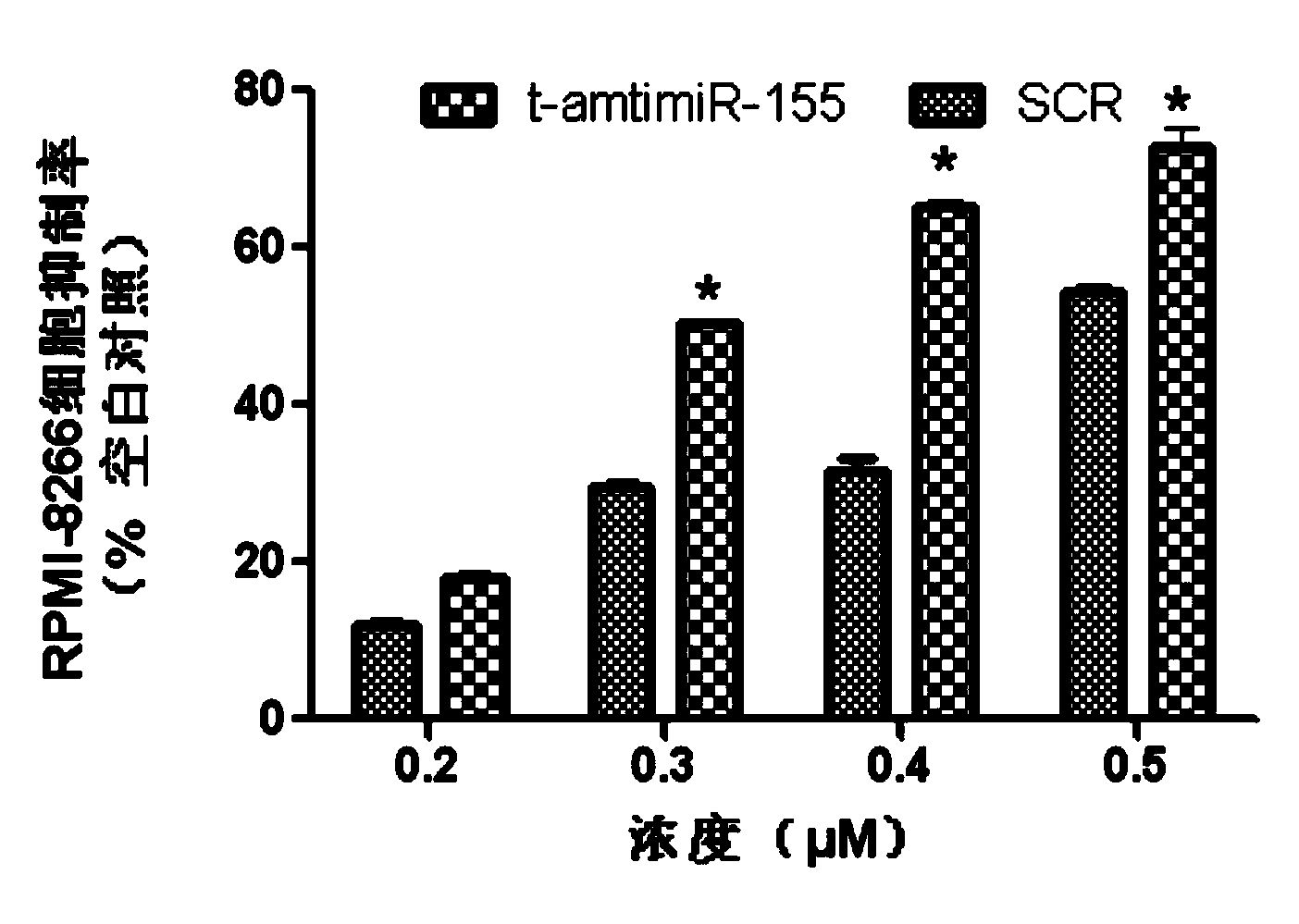
Antisense oligodeoxynucleotide for micro RNA-155 seed sequence and application
InactiveCN103882020AInhibit progressPrevent proliferationGenetic material ingredientsAntineoplastic agentsAntisense nucleic acidApoptosis
The invention discloses an antisense oligodeoxynucleotide for a micro RNA-155 seed sequence and an application. Micro antisense nucleic acids t-antimiR-155 of eight basic groups are designed and synthesized for the miR-155 seed sequence. T-antimiR-155 transfection cell test shows that t-antimiR-155 is targeted to miRNA-155 of a RPMI-8266 cell strain, so that cell growth is remarkably inhibited and apoptosis is remarkably increased. The test shows that t-antimiR-155 can effectively inhibit growth of lymphoma cells by interfering expression of the miRNA-155, and meanwhile, the test shows that the miRNA-155 can probably be taken as a potential target spot for gene therapy of lymphoma. The t-antimiR-155 can be used for preparing anti-tumor medicines, and particularly medicines for treating multiple myeloma.
Owner:JINAN UNIVERSITY
Preparation method and application of tilapia mossambica-derived streptococcus agalactiae recombinant CBP protein vaccine
InactiveCN106834308AEasy to storeHigh standardAntibacterial agentsBacterial antigen ingredientsDiseaseStreptococcus agalactiae
The invention discloses a preparation method and application of a tilapia mossambica-derived streptococcus agalactiae recombinant CBP protein vaccine. The recombinant CBP protein vaccine is prepared from tilapia mossambica-derived streptococcus agalactiae recombinant CBP protein. The amino acid sequence of the tilapia mossambica-derived streptococcus agalactiae recombinant CBP protein is shown as a sequence 2. Tilapia mossambica-derived streptococcus agalactiae is used as a research object and is cloned into a Cbp gene from a tilapia mossambica-derived streptococcus agalactiae genome; the tilapia mossambica-derived streptococcus agalactiae is connected with a pET28a(+) carrier to construct an expression vector, and prokaryotic expression and purification are carried out in E.coliBL21(DE3), so as to obtain the recombinant CBP protein vaccine with high specificity and immune protective efficacy of 56.35 percent+ / -8.09 percent; a foundation is laid for immune prevention and treatment of tilapia mossambica streptococcus agalactiae diseases.
Owner:SUN YAT SEN UNIV
Purification technique of biodegradable polyesters
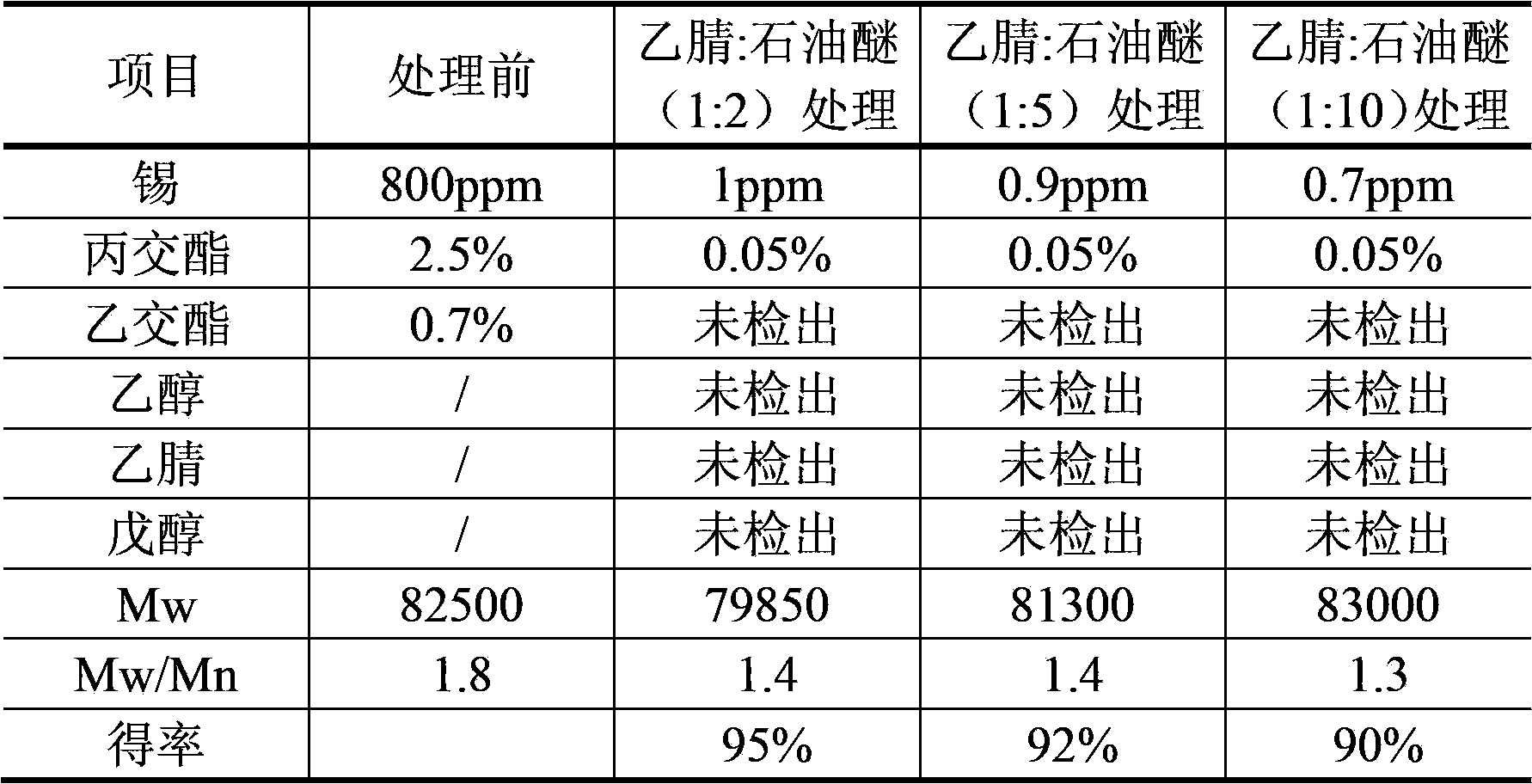
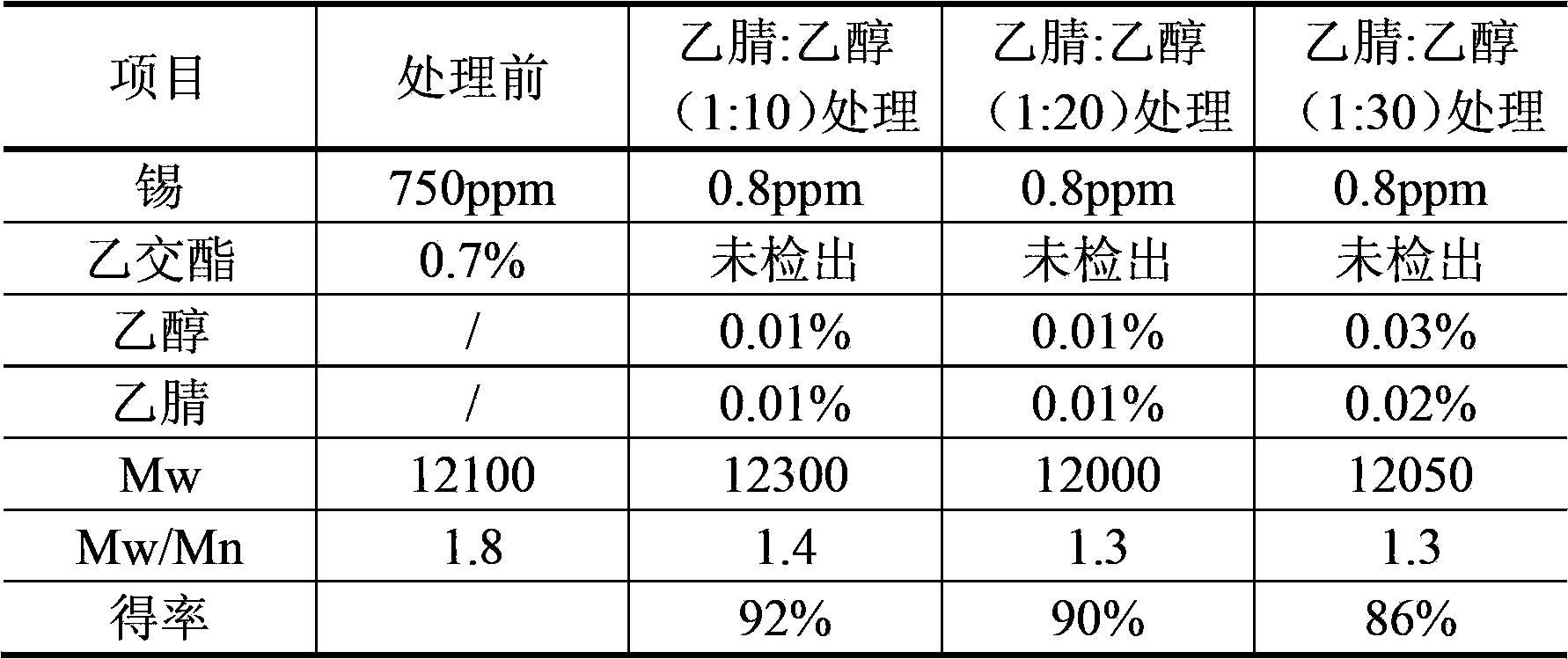
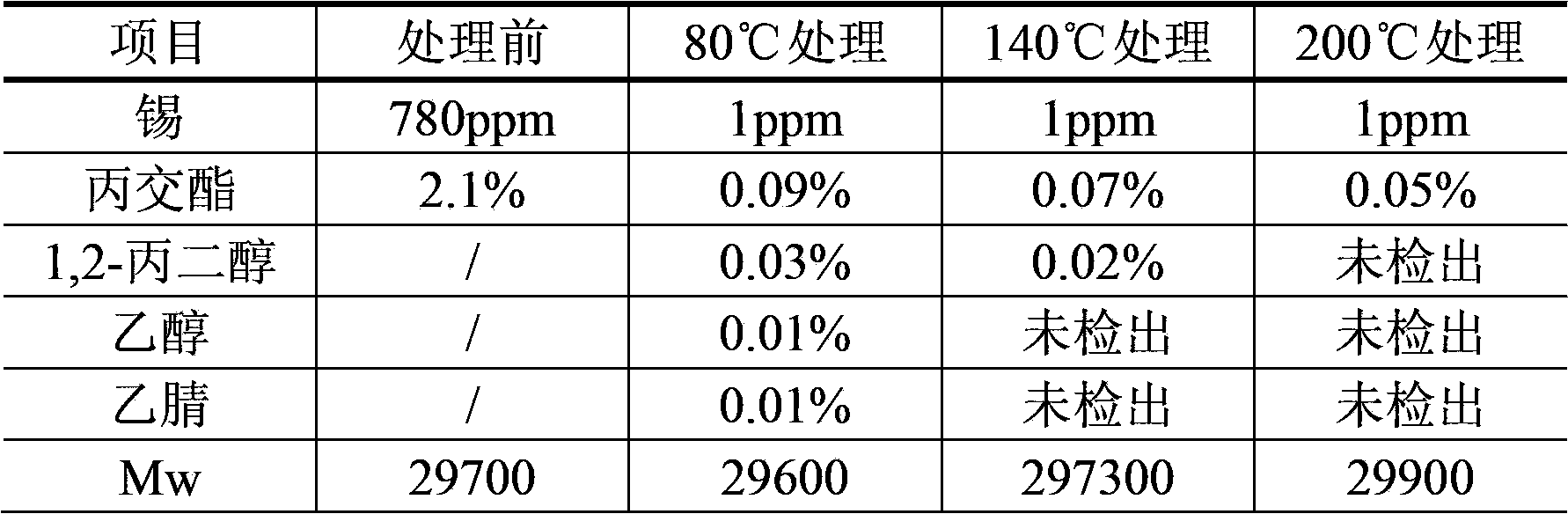
The invention discloses a purification technique of biodegradable polyesters. The purification technique comprises: (1) mixing an organic solvent with the polyesters to prepare a solution containing the polyesters and having a concentration of the polyesters between 0.05 to 8 g / 100 ml; (2) mixing an extractant with the solution and performing extraction, separating an organic phase which contains the polyesters, and concentrating the organic phase to obtain a concentrate; and (3) mixing the concentrate with a precipitant and stirring, and drying after liquid-solid separation. The organic solvent used in step (1) is acetonitrile, N,N-dimethylformamide or N,N-dimethylacetamide; the extractant used in step (2) is an alkane, the number of carbon atoms in which is between 5 and 8; and the volume ratio of the organic solvent to the extractant in step (1) is between 1:2 and 1:10. The technique can effectively remove catalysts, oligomers, monomers and the organic solvent in the biodegradable polyesters, so that high quality biodegradable polymer particles can be obtained.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT
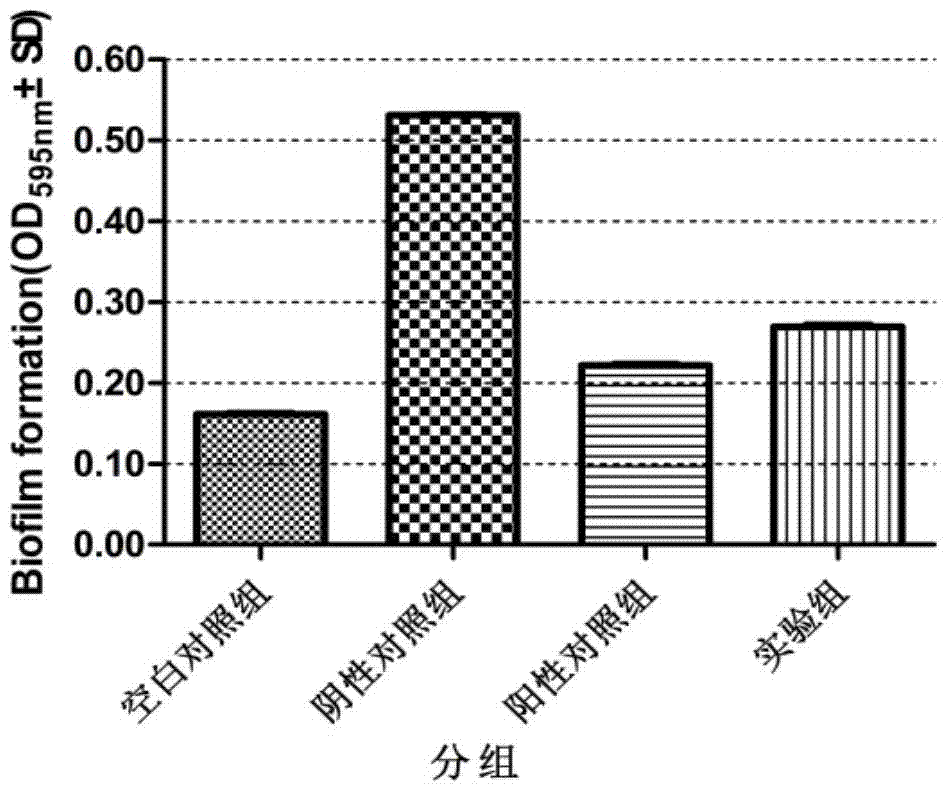
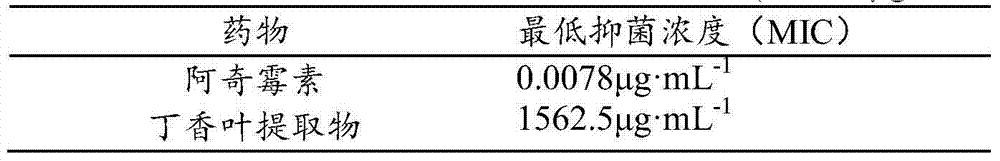
Application of clove leaf extractives in inhibition of streptococcus suis and intervention and elimination of streptococcus suis biofilm
InactiveCN103585277AImprove biological activityLong-lasting effectAntibacterial agentsPlant ingredientsBiofilmClove leaf extract
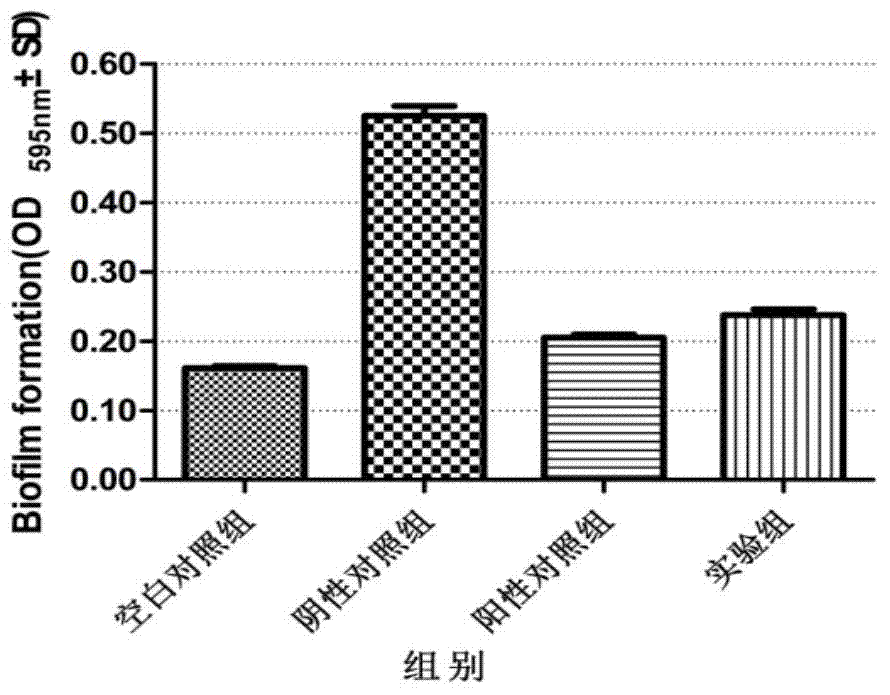
The invention discloses an application of clove leaf extractives in inhibition of streptococcus suis and intervention and elimination of a streptococcus suis biofilm. The intervention effect of the clove leaf extractives to the formation of the streptococcus suis biofilm and the elimination effect of the clove leaf extractives to the mature streptococcus suis biofilm are researched by using a crystal violet method and a scanning electron microscope. An experiment result proves that the clove leaf extractives have a remarkable intervention effect on the formation of the streptococcus suis biofilm and a remarkable elimination effect for the mature streptococcus suis biofilm, so that the clove leaf extractives can be used for inhibiting the adhesion of the streptococcus suis and the formation of the streptococcus suis biofilm and can take the elimination effect on the mature biofilm. The invention provides a novel treating approach for relevant infections caused by the streptococcus suis biofilm.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Pellet type pantoprazole sodium enteric capsule and preparation method thereof
InactiveCN104644616AImprove stabilityAvoid photolysisOrganic active ingredientsDigestive systemGranularityIsolation layer
The invention discloses a pellet type pantoprazole sodium enteric capsule and a preparation method thereof. The pellet type pantoprazole sodium enteric capsule is a sustained-release preparation composed of a capsule shell and uniformly mixed pantoprazole sodium pellets held in the capsule shell; the pantoprazole sodium pellet from inside to outside in turn comprises a blank pellet core, a main drug layer, an isolation layer and an enteric layer, wherein the weight of the main drug layer is 14-55% of that of the blank pellet core, the weight of the isolation layer is 9-10% of that of the total weight of the blank pellet core and the main drug layer, the weight of the enteric layer is 28-32% of that of the total weight of the blank pellet core, the main drug layer and the isolation layer; 40-60% of that of the pantoprazole sodium pellet is coated with a pigmented layer on the outside of the enteric layer; the weight of the pigmented layer is 0.5-1% of that of the total weight of the blank pellet core, the main drug layer, the isolation layer and the enteric layer; the diameter of the blank pellet core is 0.8-1.0 mm; the granularity of the pantoprazole sodium pellet is 14-20 mesh; each capsule contains 20-40 mg of the pantoprazole sodium. The pellet type pantoprazole sodium enteric capsule has the advantages of acidproof, rapid release and stable.
Owner:ZHEJIANG CHANGDIAN PHARMA +1
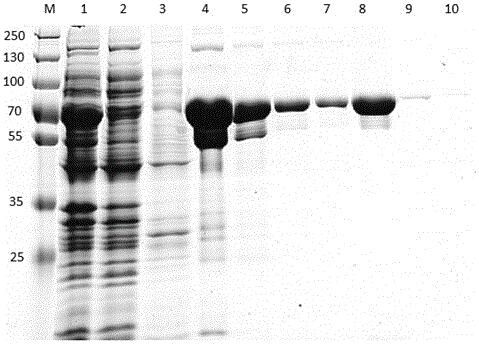
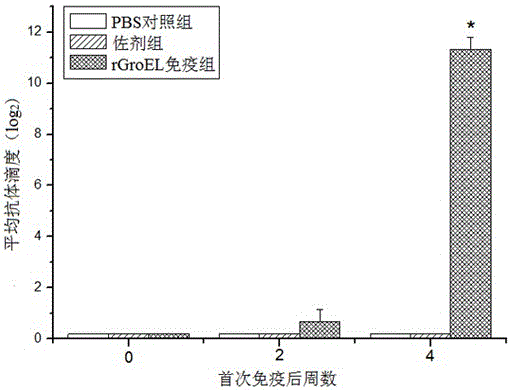
Preparation method and application of tilapia source streptococcus agalactiae recombinant GroEL protein vaccine
ActiveCN105861521ALess side effectsEasy to storeAntibacterial agentsBacterial antigen ingredientsStreptococcus mastitidisTGE VACCINE
The invention discloses a preparation method and application of a tilapia source streptococcus agalactiae recombinant GroEL protein vaccine. The recombinant GroEL protein vaccine is prepared by tilapia source streptococcus agalactiae recombinant GroEL protein. An amino acid sequence of the tilapia source streptococcus agalactiae recombinant GroEL protein is as shown in SEQ ID NO.2. In the invention, tilapia source streptococcus agalactiae is taken as a research object, a GroEL gene is obtained from a tilapia source streptococcus agalactiae genome by cloning, the cloned GroEL gene is connected with a pET28a(+) carrier to construct an expression vector, and the vector is subjected to prokaryotic expression and purification in E.coli BL21(DE3) to obtain the recombinant GroEL protein vaccine with strong specificity and an immune protective efficacy of 68.61+ / -7.39 percent, so that a foundation is laid for immune prevention and treatment of a tilapia streptococcus agalactiae disease.
Owner:SUN YAT SEN UNIV
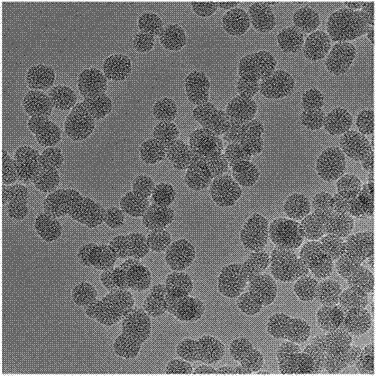
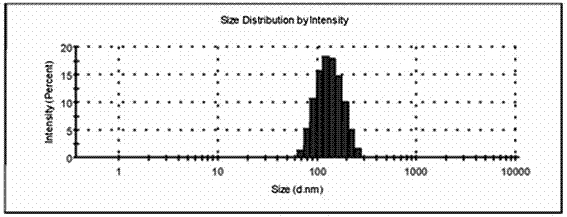
Mesoporous silica-lactobionic acid targeted nanoparticles loaded with Sorafenib/siRNA
ActiveCN107349432AImprove stabilityImprove efficiencyGenetic material ingredientsPharmaceutical non-active ingredientsElectrostatic adsorptionNormal cell
The invention discloses mesoporous silica-lactobionic acid targeted nanoparticles loaded with Sorafenib / siRNA and an application of the mesoporous silica-lactobionic acid targeted nanoparticles loaded with the Sorafenib / siRNA in preparing of anticancer therapy drugs. The targeted nanoparticles are prepared through the steps that surface amination modifying is conducted on mesoporous silica nanoparticles; then inner pore channels of the mesoporous silica nanoparticles are internally loaded with the Sorafenib; and lactobionic acid is covalently coupled to the outer surfaces of the mesoporous silica nanoparticles, and the siRNA is adsorbed on the outer surfaces of the mesoporous silica nanoparticles through the electrostatic adsorption effect. According to the drug loading system, the stability of the siRNA can be improved, and targeted drug delivery of the Sorafenib can be further achieved, so that the toxic and side effects of the mesoporous silica-lactobionic acid targeted nanoparticles on normal cells are effectively relieved.
Owner:FUZHOU UNIVERSITY
Strychnine gel preparation and preparation method thereof
PendingCN112386565AToxic and side effectsImprove bioavailabilityOrganic active ingredientsAntipyreticGel preparationIrritation
The invention belongs to the field of pharmaceutical preparations, and particularly relates to a strychnine gel preparation and a preparation method thereof. The gel preparation comprises 0.5%-1% of strychnine, 0.5%-3% of a gel skeleton, 10%-30% of a cosolvent and the balance of water. According to the prepared strychnine hydrogel, simple pharmaceutic adjuvants are used, no transdermal enhancer isused, but the transdermal effect is excellent, and the strychnine hydrogel is easy to spread, good in biocompatibility, good in skin absorption, good in drug membrane adhesiveness and free of irritation to skin and mucous membranes.
Owner:BEIJING INCREASEPHARM CORP LTD
Anthocyanin-contained composition and application thereof
InactiveCN113143908AStrong reductionEnhanced Antioxidant MechanismOrganic active ingredientsPowder deliveryEscherichia coliSuperoxide
The invention relates to the field of medicinal use of anthocyanin and discloses an anthocyanin-contained composition and application thereof. If anthocyanin is extracted from grape skin, the composition is applied to antioxidation drugs, plays a role in clearing away DPPH free radicals, superoxide anion free radicals (O2<->.) and hydroxyl free radicals (.OH) and has a relatively high reducing property; if the anthocyanin is extracted from mulberries, the composition is applied to bacteriostatic drugs and plays a role in inhibiting staphylococcus aureus, Escherichia coli and bacillus subtilis; and the composition can also be applied to blood sugar reducing, blood fat reducing and atherosclerosis inhibiting and plays a role in inhibiting intestinal alpha-glucosidase, the blood sugar reducing activity of acarbose is enhanced obviously, the synthesis of fats is inhibited, the decomposition of the fats is stimulated, and the composition has blood sugar reducing and blood fat reducing actions and can be used for inhibiting expression of adhesion molecules and inhibiting formation of atherosclerosis.
Owner:HUAIYIN INSTITUTE OF TECHNOLOGY
Mini-pill type nicergoline capsule and preparation method thereof
InactiveCN104622850AImprove solubilityWidely distributedSenses disorderNervous disorderSide effectBiomedical engineering
The invention discloses a mini-pill type nicergoline capsule and a preparation method thereof. The mini-pill type nicergoline capsule is a sustained-release preparation and is prepared from a capsule shell and a nicergoline mini-pill accommodated in the capsule shell, wherein the nicergoline mini-pill sequentially comprises an empty pill core, a main drug layer and a pigmented layer from inside to outside; the main drug layer accounts for 15-60 percent of the empty pill core, and the pigmented layer accounts for 0.5-1 percent of the total weight of the empty pill core and the main drug layer. The mini-pill type nicergoline capsule has the advantages of small stimulation to intestines and stomach, high utilization rate, high stability, less toxic or side effects and capability of fully releasing effective components in time.
Owner:ZHEJIANG CHANGDIAN PHARMA +1
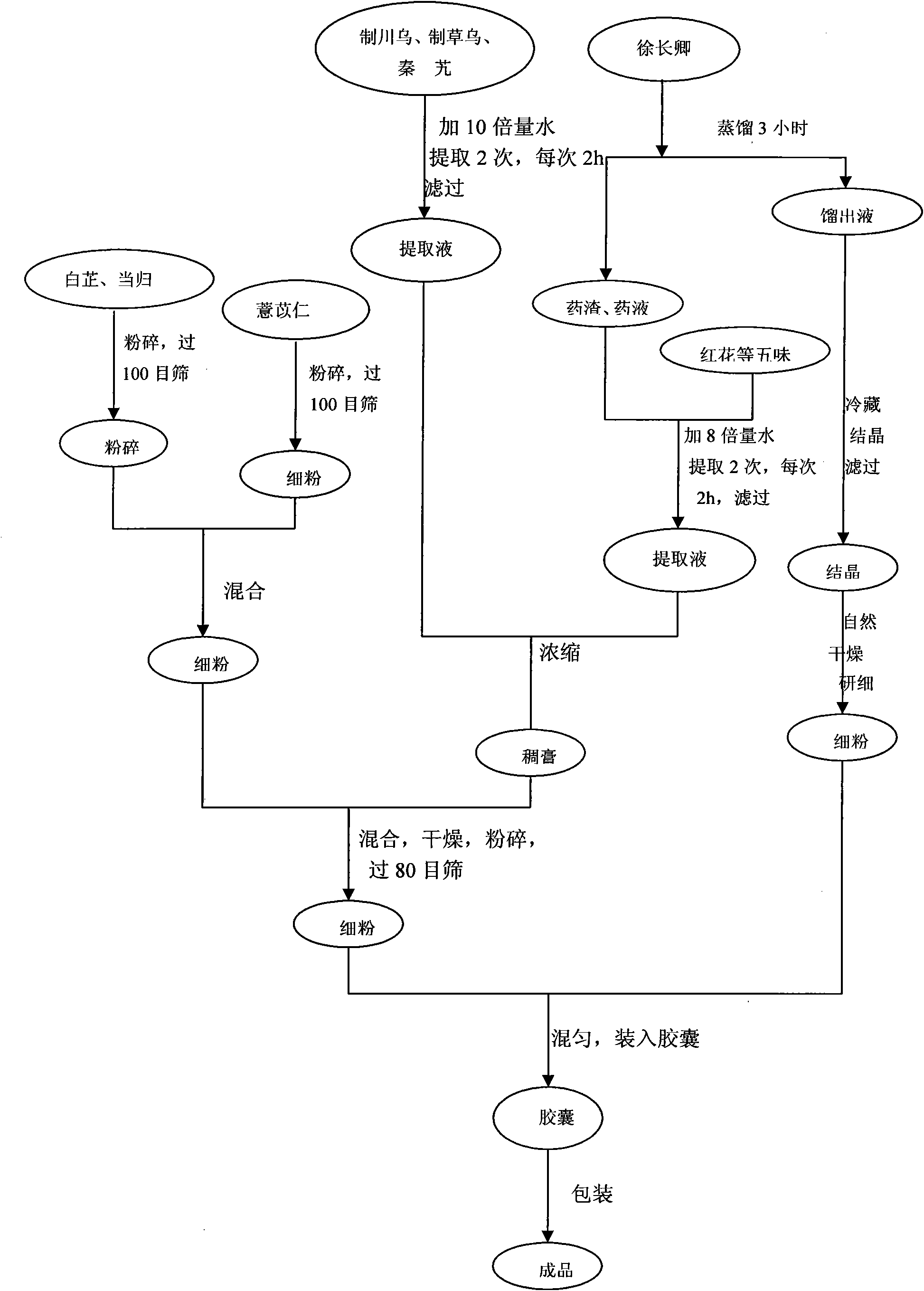
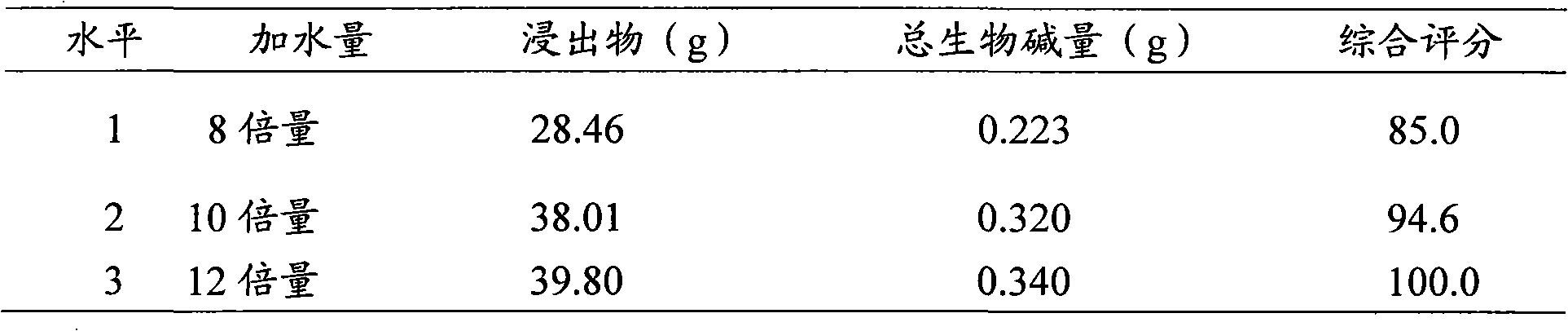
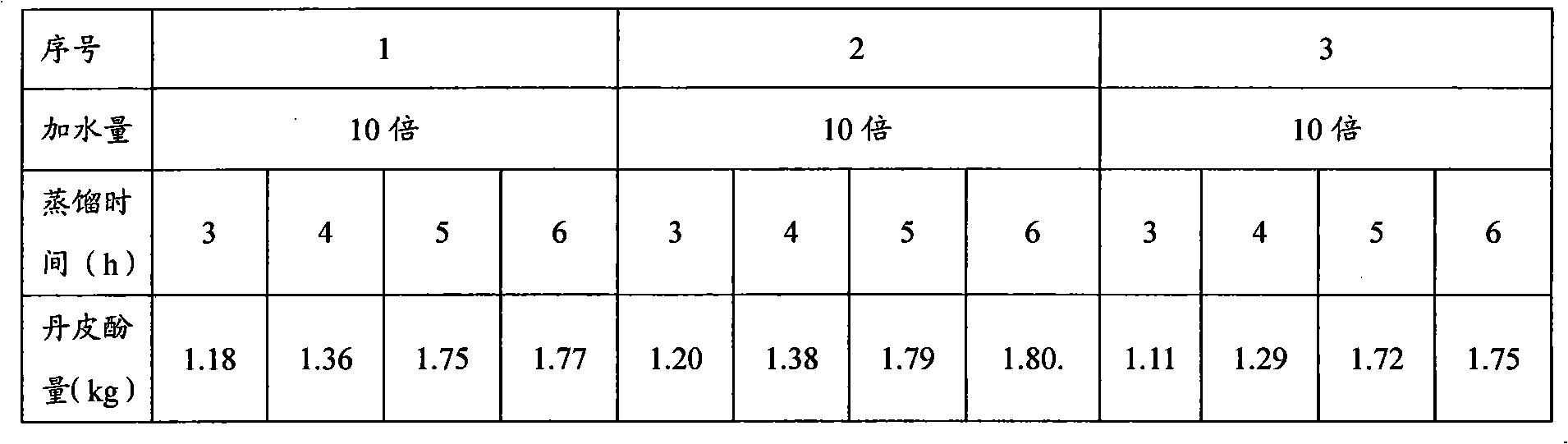
Production method of bone spur pain-eliminating capsule
ActiveCN101856472AHigh yieldToxic and side effectsNervous disorderAntipyreticMedicineEconomic benefits
The invention relates to a production method of a bone spur pain-eliminating capsule, which comprises the steps of treating dahurian angelica root, Chinese angelica and semen coicis, treating radix aconiti preparata, radix aconiti agrestis praeparata and gentiana macrophylla, treating paniculate swallowwort root and other steps. By adopting the method, the yield of paeonol in the paniculate swallowwort root is greatly increased, the water consumption during extraction is greatly reduced, the distillation time is greatly shortened and the energy consumed for extraction is remarkably reduced and the utilization rate of the equipment is remarkably improved. Therefore, the invention has unexpected technical effects, remarkable economic benefits and wide application prospect.
Owner:中山市恒生药业有限公司
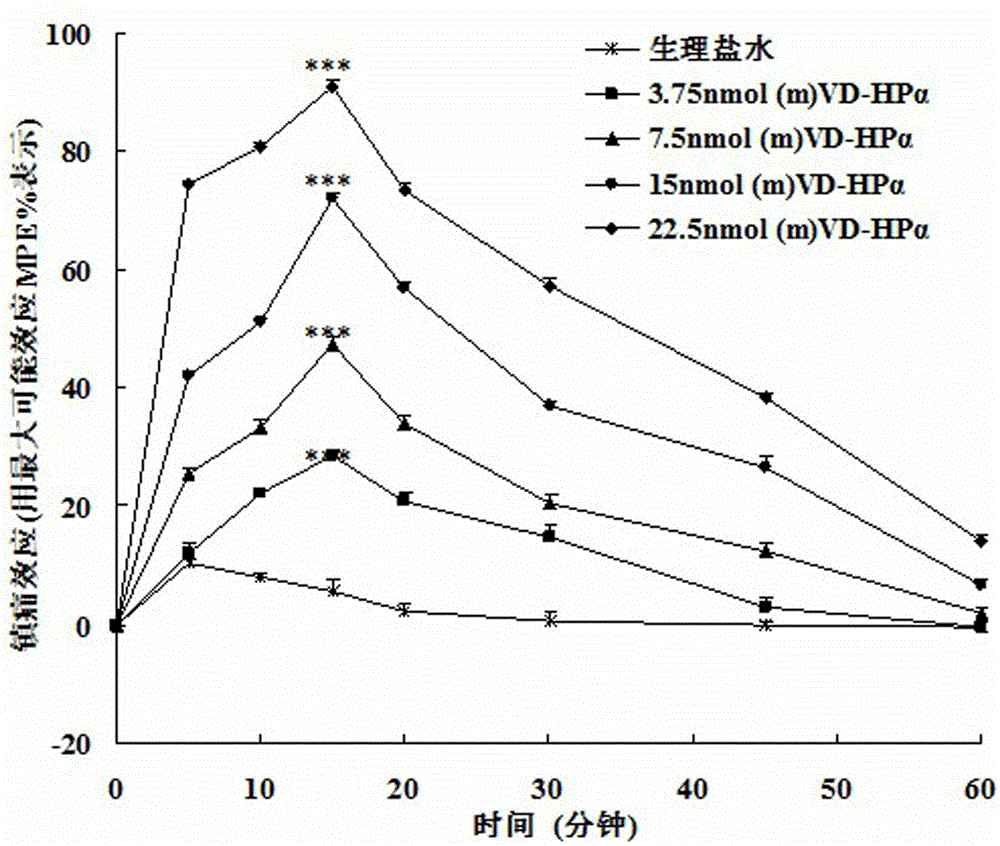
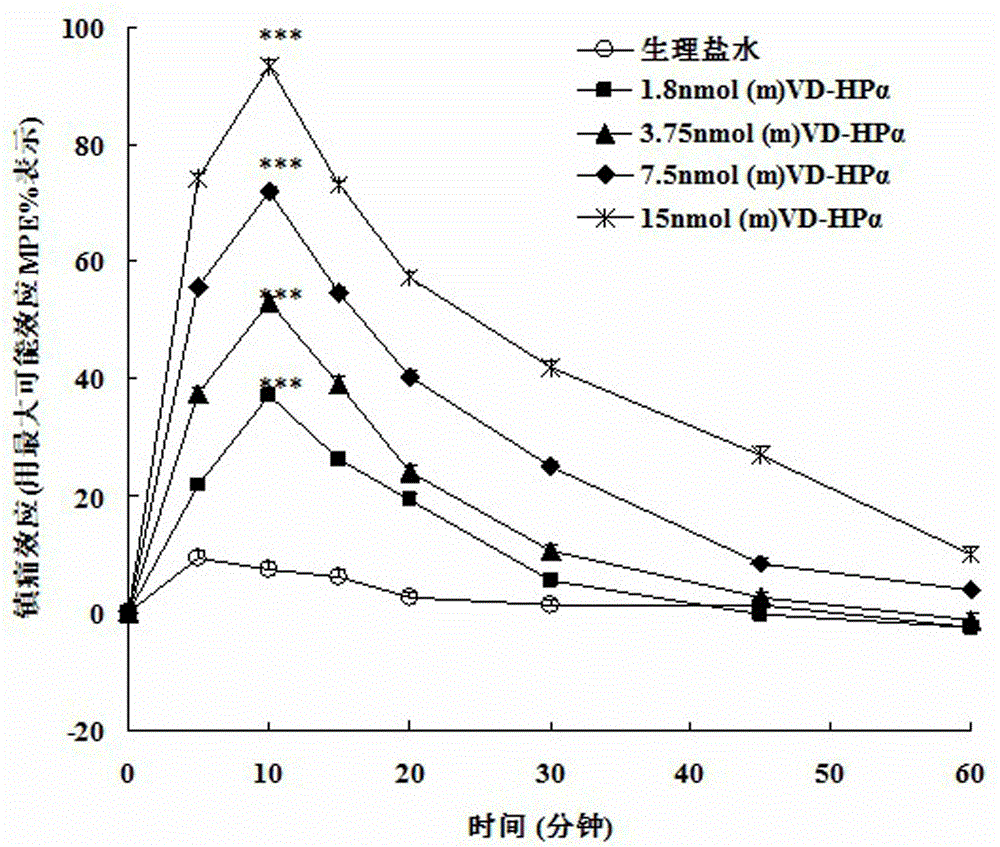
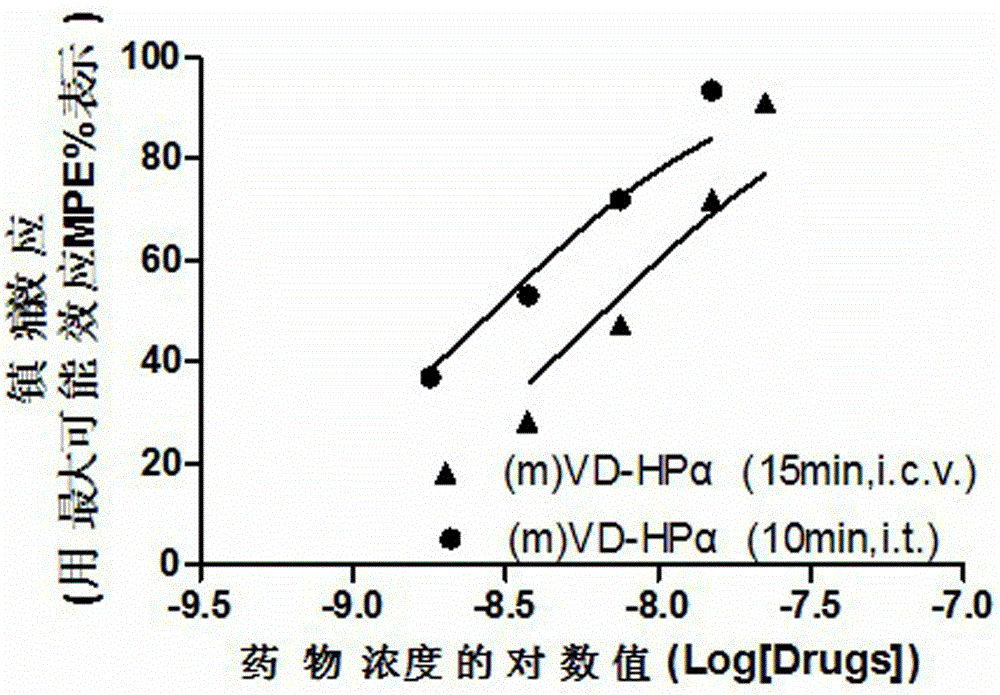
Application of endogenic marihuana peptide agonist (m) VD-Hp alpha in preparing analgesics
ActiveCN103142996AToxic and side effectsImprove solubilityPeptide/protein ingredientsAntipyreticSide effectPeptide ligand
The invention discloses application of endogenic marihuana peptide agonist (m) VD-Hp alpha in preparing analgesics, which means that an injection preparation or lyophilized powder preparation is prepared from the endogenic marihuana peptide agonist (m) VD-Hp alpha serving as an active ingredient and auxiliary materials according to a conventional process of pharmaceutics. A series of in-vivo activity detections indicate that marihuana endogenous peptide ligand (m) VD-Hp alpha has strong central analgesia activity, and has an advantage of low central side effect, which means that the marihuana endogenous peptide ligand (m) VD-Hp alpha seldom has the side effects of constipation and hypokinesia in an effective analgesic dose range, has a small influence on body temperature, and is slow in the analgesic tolerance process. Therefore, the endogenic marihuana peptide agonist (m) VD-Hp alpha has a good application prospect in preparing high-efficiency low-side-effect analgesics.
Owner:SHANGHAI TIANCI LIFE SCI DEV CO LTD
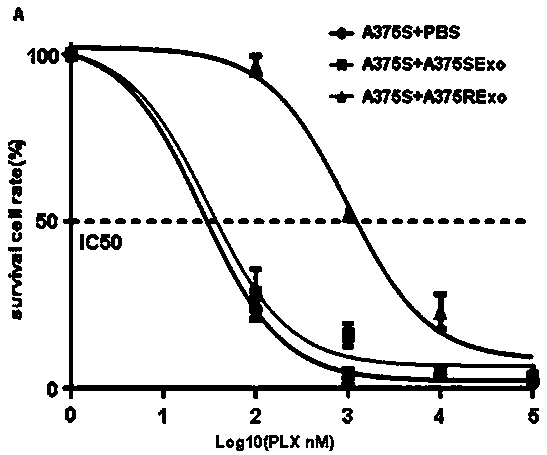
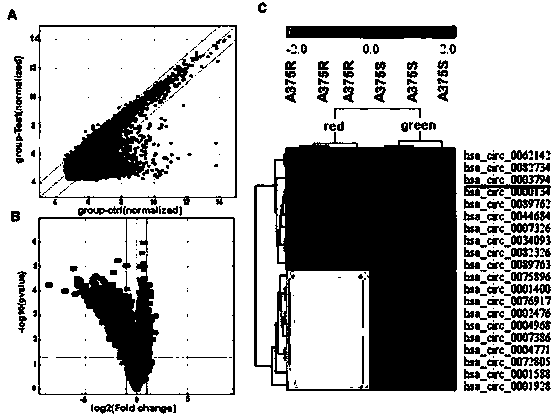
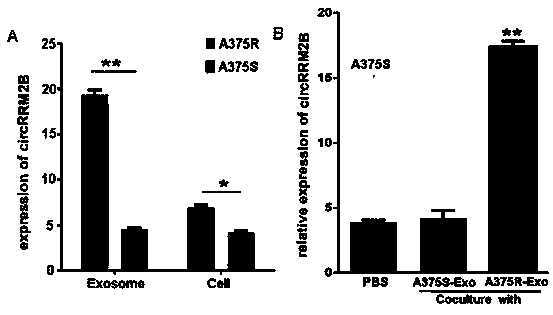
Applications of circRRM2B gene as new target in screening Vemurafenib-resistant melanoma treatment drugs
ActiveCN111206101AToxic and side effectsSignificant technological progressMicrobiological testing/measurementAntineoplastic agentsPharmaceutical drugVemurafenib
The invention provides applications of a circRRM2B gene as a new target in screening Vemurafenib-resistant melanoma treatment drugs. The drugs take the circRRM2B gene as a target and inhibit or silence the expression of the circRRM2B gene. Applications of reagents for inhibiting or silencing the expression of the circRRM2B gene in preparing drugs for inhibiting melanoma are also provided. Throughchip screening, the circRRM2B is found being related to the Vemurafenib resistance of the melanoma and is the new target to screen the Vemurafenib-resistant melanoma treatment drugs, so that the circRRM2B gene has positive significance on screening new drugs. Meanwhile, the discovery of the new target provides a new thought for the treatment of drug-resistant melanoma.
Owner:SHANGHAI EAST HOSPITAL EAST HOSPITAL TONGJI UNIV SCHOOL OF MEDICINE
Pellet type omeprazole enteric capsule and preparation method thereof
InactiveCN104546737AWidely distributedIncrease local concentrationOrganic active ingredientsDigestive systemGranularityIsolation layer
The invention discloses a pellet type omeprazole enteric capsule and a preparation method thereof. The pellet type omeprazole enteric capsule is a sustained-release preparation, and is composed of a capsule shell and evenly mixed omeprazole pellets received in the capsule shell; each omeprazole pellet is composed of a blank pill core, a main drug layer, an isolation layer and an enteric layer in order from inside to outside, wherein the weight of the main drug layer accounts for 15-60% of that of the blank pill core, the weight of the isolation layer accounts for 10% of the total weight of the blank pill core and the main drug layer, the weight of the enteric layer accounts for 28-32% of the total weight of the blank pill core, the main drug layer and the isolation layer; besides, the enteric layers of 40-60% of omeprazole pellets are further coated with pigment layers; the weight of the pigment layer accounts for 0.5-1% of the total weight of the blank pill core, the main drug layer, the isolation layer and the enteric layer; the diameters of the blank pill cores are 0.8-1.0mm; the granularity of the omeprazole pellets is 14-20 meshes; each capsule contains 15-30mg of omeprazole. The pellet type omeprazole enteric capsule has the advantages of good acid resistance, sufficient active ingredient release without delay, good stability and the like.
Owner:ZHEJIANG CHANGDIAN PHARMA +1
Preparation method of medicinal and edible fermented beverage with enteritis relieving effect
PendingCN112167491AMany complicationsToxic and side effectsAntipyreticAnalgesicsBiotechnologyPolygonum fagopyrum
The invention relates to the field of food enzymes, and particularly relates to a preparation method of a medicinal and edible fermented beverage with an enteritis relieving effect. The product formula is composed of three parts, namely five-cereal raw materials, medicinal and edible raw materials and compound auxiliary materials, wherein the five-cereal raw materials comprise a mixture of Chineseoats, buckwheat, millet and soybeans; and the medicinal and edible raw materials are a mixture of honeysuckle, bighead atractylodes rhizome, liquorice and dried tangerine or orange peel. The preparation method comprises the following steps: pretreating the raw materials, adding lactobacillus plantarum and lactobacillus rhamnosus, performing static fermentation for 5-7 days, centrifuging, collecting the supernatant, performing secondary extraction on the precipitate by adopting boiling water reflux, merging the fermentation clear solution and water extraction clear solution, concentrating to 1 / 2 volume, adding the compound formula raw materials, stirring and dissolving, sequentially adding a clarifying agent and a preservative, filling and performing pasteurization, thereby obtaining the product. According to the preparation method disclosed by the invention, the toxic and side effects of the medicines are reduced, the blood sugar reducing function is enhanced, the fermentation efficiency is improved, the body immunity is improved, the flavor is adjusted by adopting an after-ripening compound formula, the taste is improved, and an enzyme beverage with a blood sugar reducing effectand excellent taste is formed.
Owner:JINAN HANGCHEN BIOTECHNOLOGY CO LTD +2
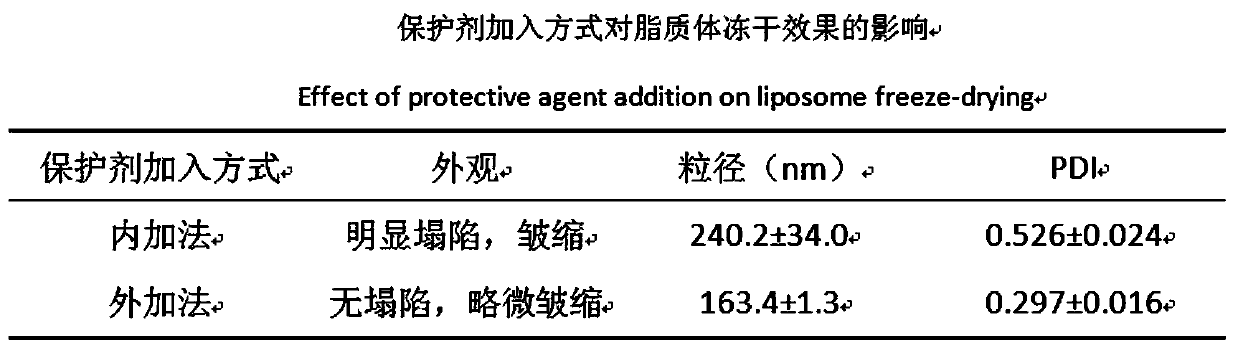
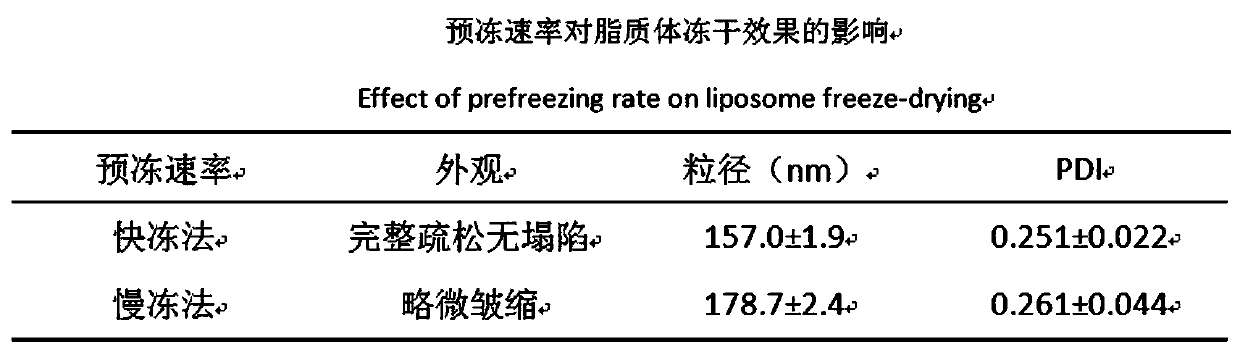
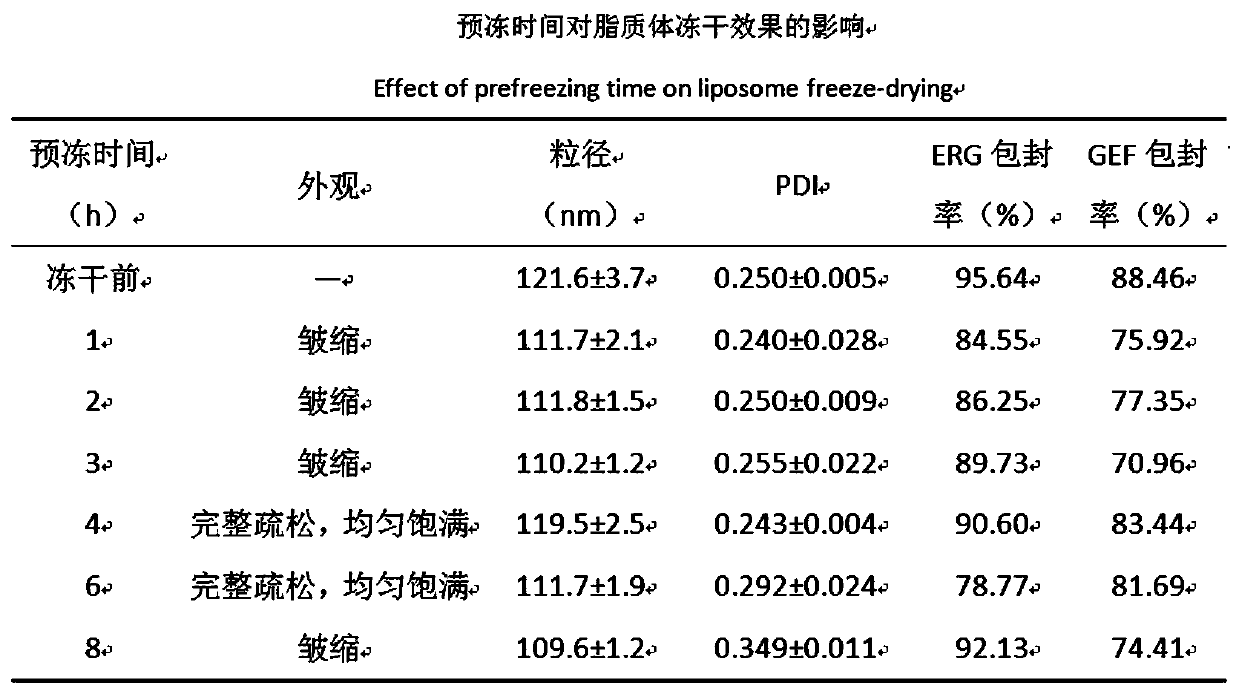
Preparation method of ergosterol and gefitinib combined compound liposome freeze-dried powder, liposome and application thereof
ActiveCN110623964AStrong proliferation inhibitory effectGood apoptosis rateOrganic active ingredientsPowder deliveryCyclic peptideFluorescence
The invention relates to a preparation method of RGD cyclic peptide R8 peptide modified ergosterol and gefitinib combined compound liposome freeze-dried powder. The preparation method comprises the following steps: adding a freeze-drying protective agent into a pre-prepared RGD / R8-ERG / GEF-LIP liposome suspension in an external addition manner; and finally, preparing the freeze-dried powder of thecompound liposome by adopting a freeze-drying method. The RGD / R8-ERG / GEF-LIP lipidosome suspension is prepared by adopting the following method: firstly, preparing ERG / GEF-LIP, and then, preparing theRGD / R8-ERG / GEF-LIP lipidosome suspension by adopting a post-insertion method. According to the invention, an RGD / R8-ERG / GEF-LIP active drug-loading liposome drug delivery system is successfully constructed; ERG / GEF-LIP is used for investigating a freeze-drying process and a prescription, and after an optimal prescription process is screened out, the optimal prescription process is applied to RGD / R8-ERG / GEF-LIP for verification. An in-vitro test result of RGD / R8-ERG / GEF-LIP freeze-dried powder proves that the RGD / R8-ERG / GEF-LIP freeze-dried powder has a relatively strong tumor cell proliferation inhibition effect, the fluorescence uptake intensity and the good cell apoptosis rate.
Owner:ZHEJIANG CHINESE MEDICAL UNIVERSITY
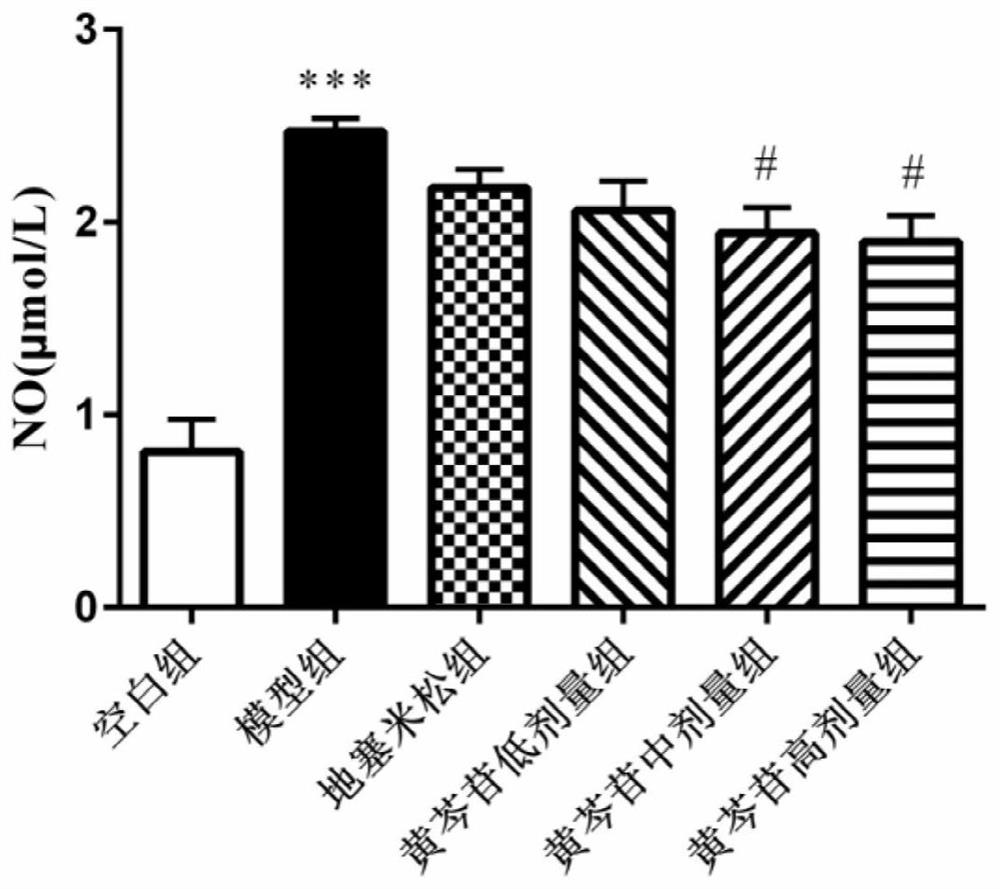
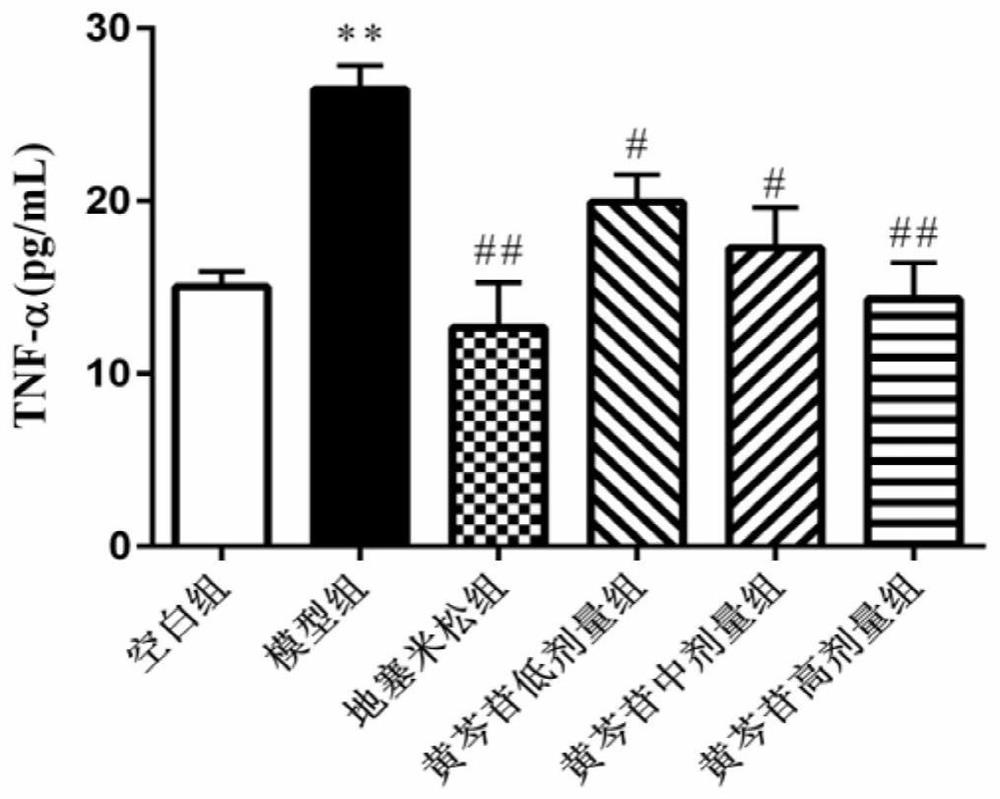
Application of baicalin in preparation of medicine for preventing and/or treating asymptomatic hyperuricemia and/or uric acid nephropathy
InactiveCN112451537AImprove toleranceImprove complianceOrganic active ingredientsSkeletal disorderXanthineRenal Tubular Epithelial Cells
The invention belongs to the field of biological medicines, and relates to an application of baicalin in preparation of a medicine for preventing and / or treating asymptomatic hyperuricemia and / or uricacid nephropathy. Based on animal and cell experiments, the effect of baicalin on hyperuricemia nephropathy is studied, and the result shows that baicalin can inhibit the activity of xanthine oxidase, inhibit uric acid generation, reduce the serum uric acid level of mice with uric acid nephropathy and remarkably improve the renal function of the mice. The baicalin inhibits damage of sodium uratecrystals to rat renal tubular epithelial cells by reducing secretion of TNF alpha, IL-1beta, lactate dehydrogenase and NO.
Owner:WUHAN POLYTECHNIC UNIVERSITY
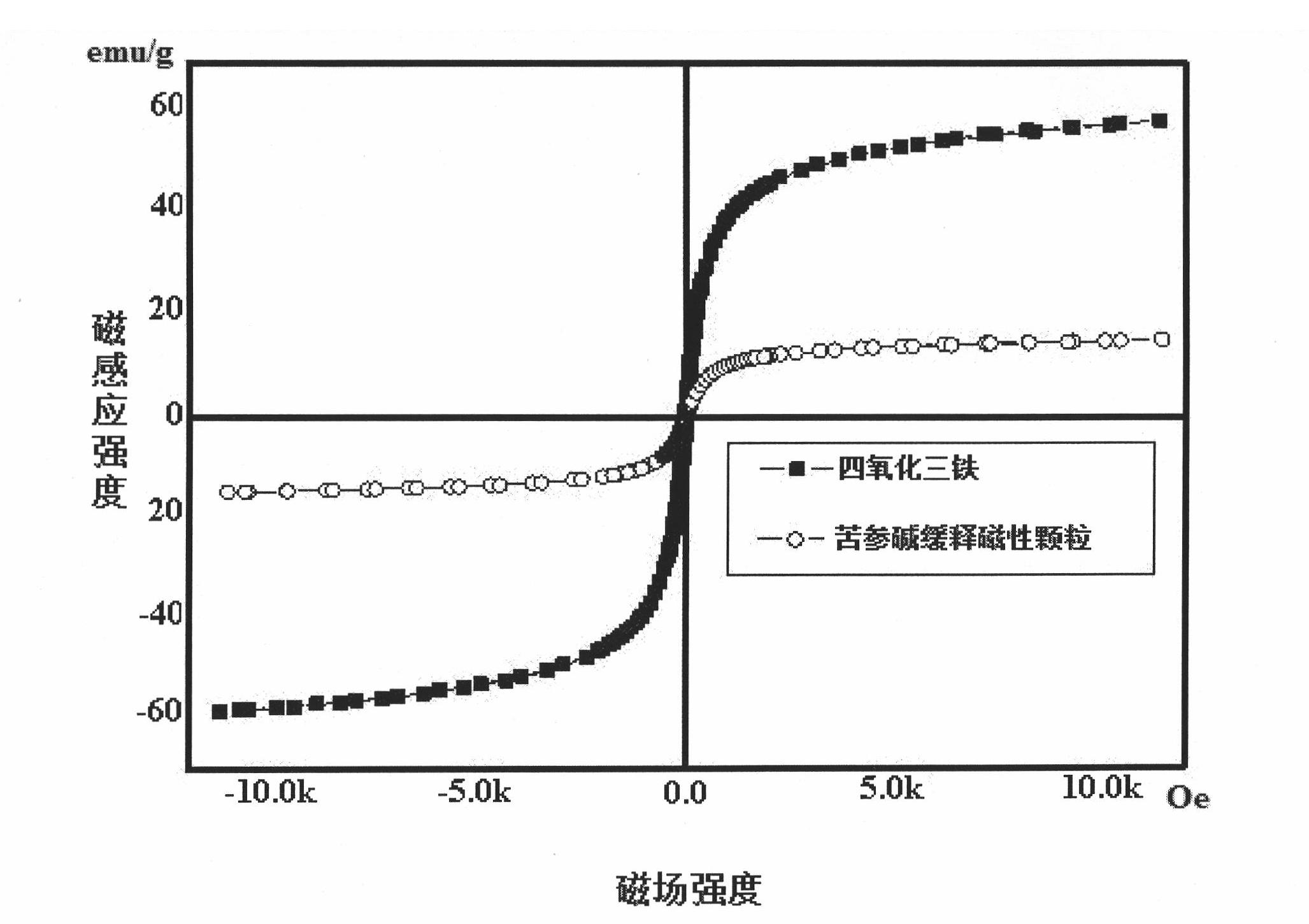
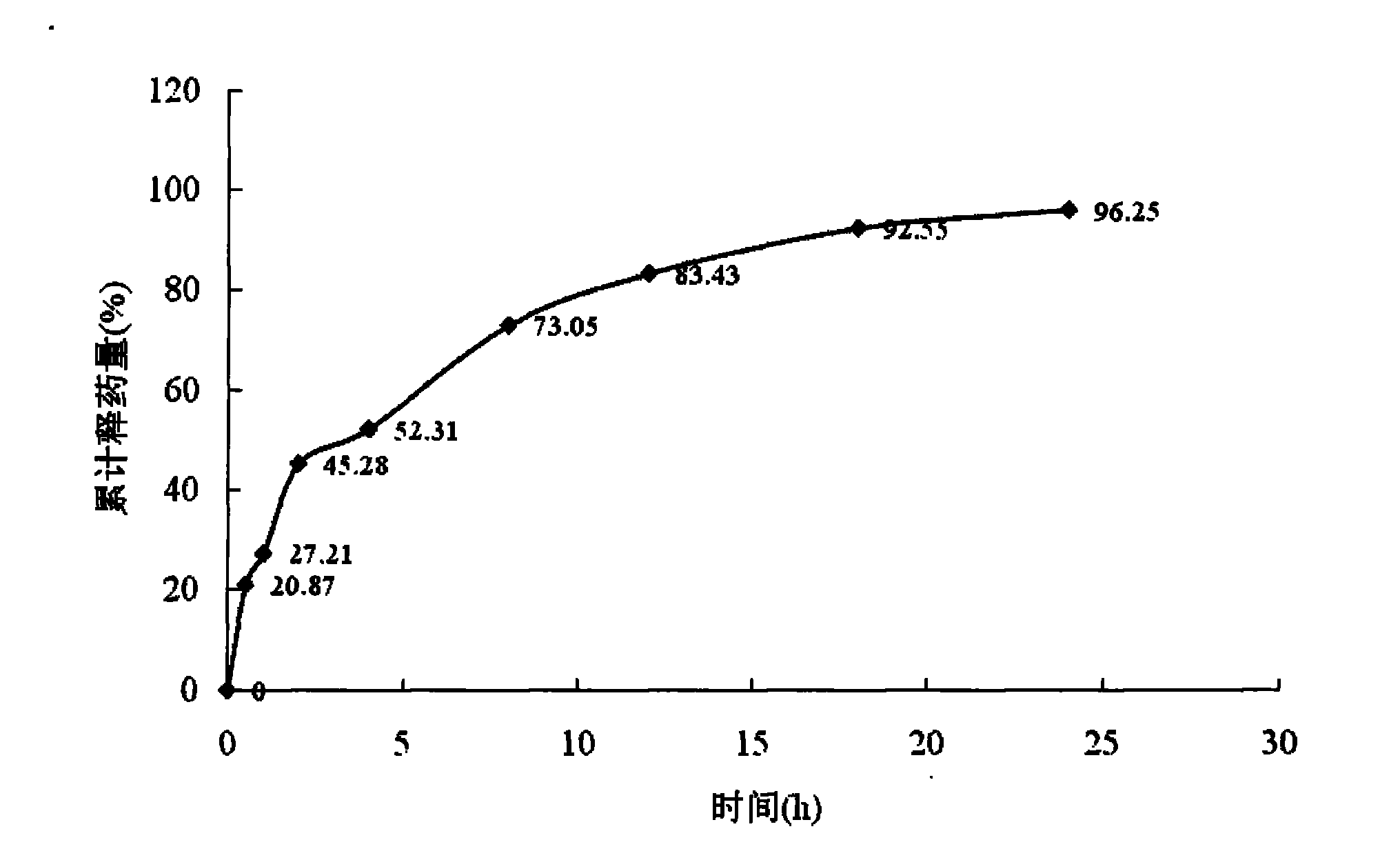
Matrine magnetic slow-releasing capsule and preparation method
InactiveCN102302473ASustained releaseRelease constantOrganic active ingredientsDigestive systemSide effectMass ratio
The invention discloses a matrine magnetic slow-releasing capsule for treating gastrointestinal tumors through oral administration, and a preparation method of the matrine magnetic slow-releasing capsule. The content of the matrine magnetic slow-releasing capsule is matrine magnetic slow-releasing particles; the matrine magnetic slow-releasing capsule comprises drug carried magnetic particles and a slow-releasing coating film; the drug carried magnetic particles of the content include matrine, magnetic substance (ferroferric oxide) nanoparticles, microcrystalline cellulose and chitosan in the slow-releasing coating film; and the mass ratio of the matrine, the magnetic substance (ferroferric oxide) nanoparticles, the microcrystalline cellulose and the chitosan is 1 : 0.5-2 : 1-8: 0.1-3 . Through oral administration and being guided by the magnetic field, the matrine magnetic slow-releasing capsule not only can target the gastrointestinal tumor cells, but also can delay the release speed of the main drug; the adverse effects, complications and adverse reactions of the drug can be expected to be reduced; the drug administration frequency is reduced; the compliance of the patients is increased; and the clinical treatment effect of the drug is enhanced.
Owner:LANZHOU UNIV SECOND HOSPITAL
Cataplasm for treating lumbar interveterbral disc protrusion
InactiveCN104666681APromote blood circulationImproves metabolism and nutritional statusHydroxy compound active ingredientsSkeletal disorderAchyranthes RootMENTHYL SALICYLATE
A cataplasm for treating lumbar interveterbral disc protrusion belongs to the technical field of traditional herbal medicinal preparations with undetermined structures. The cataplasm for treating lumbar interveterbral disc protrusion has the advantages of use convenience, good curative effect, fast effectiveness, no need of administration of Chinese or western medicines, no toxic side effects, and avoiding of pains of physicotherapy or operations. The cataplasm comprises the following traditional Chinese medicinal components: 10-15g of Radix Aconiti, 8-12g of Rhizoma Arisaematis, 2-6g of Rhizoma Drynariae, 8-12g of rosin, 8-12g of Rhizoma Pinelliae Preparatum, 6-10g of Chinese angelica, 8-12g of Crocus sativus, 12-18g of Radix Clematidis, 3-8g of Manchurian wildginger, 8-12g of Incised Notopterygium, 1-5g of borneol, 8-12g of camphor, 12-18g of Rheum officinale, 8-12g of peach seed, 3-8g of Semen Brassicae, 6-10g of Chinese Taxillus Twig, 10-15g of Eucommia ulmoides, 12-18g of Achyranthes bidentata, 3-8g of centipede, and also comprises the following matrix materials: 5-10% of methyl salicylate, 1-5% of sodium benzoate, 25-30% of glycerin, 1-5% of polysorbate, 10-15% of gelatin, 1-5% of carbomer, 1-5% of camphor, 1-5% of kaolin, 5-10% of sodium polyacrylate, and the balance of water.
Owner:张跃丰
5-azacytidine compound and preparation method thereof
PendingCN110746476AGuaranteed purityLess impuritiesSugar derivativesSugar derivatives preparationCombinatorial chemistryStructural formula
The invention relates to a 5-azacytidine compound and a preparation method thereof. The structural formula of the 5-azacytidine compound is shown in a formula I (shown in the specification). The compound is obtained from 5-azacytosine as a starting material by upper protection, condensation, deprotection, recondensation and a deprotection rection. Through studies of related substances, recognitionand control of an impurity profile in the 5-azacytidine compound are strengthened, the quality of a finished product is advantageously controlled, and a guarantee is provided for the safety of clinical medication. The synthesis process is simple in operation, good in yield and purity, and is environment friendly.
Owner:JIANGSU HANSOH PHARMA CO LTD
Use of coumaric acid in preparing medicine for treating human acute lymphoblastic leukemia
ActiveCN109260188AToxic and side effectsDeepen deathOrganic active ingredientsAntineoplastic agentsDrugToxicity
The invention belongs to the technical field of medicine, in particular to the use of coumaric acid in preparing a medicine for treating human acute lymphoblastic leukemia, which has the advantages oflow toxicity, small side effect, high selectivity, low price and the like.
Owner:黄嘉若 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com