Preparation method of ergosterol and gefitinib combined compound liposome freeze-dried powder, liposome and application thereof
A compound liposome and gefitinib technology, which is applied in liposome delivery, medical preparations of non-active ingredients, freeze-dried delivery, etc., can solve problems such as multidrug resistance and achieve strong tumor cell proliferation inhibition The effect of good effect and fluorescence uptake intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0051] The present invention will be further explained below in conjunction with the accompanying drawings.
[0052] This specific embodiment is only an explanation of the present invention, but not a limitation of the present invention. Any changes made by those skilled in the art after reading the description of the present invention will be protected by the patent law as long as they are within the scope of the claims.
[0053] Preparation and quality evaluation of cyclic peptide / R8 peptide modified ERG combined with GEF compound liposome freeze-dried powder
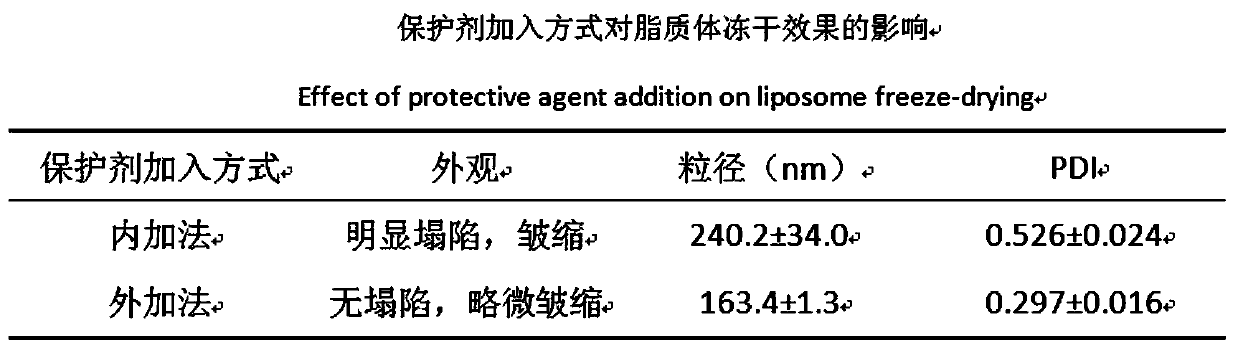
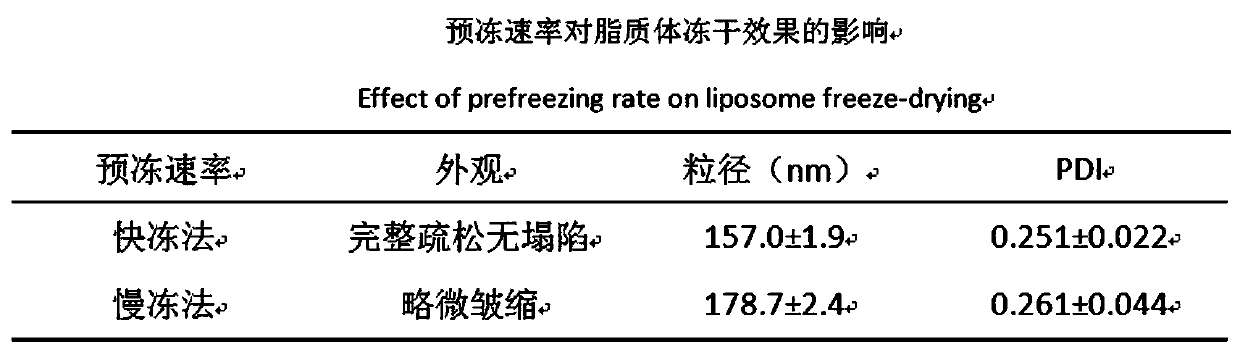
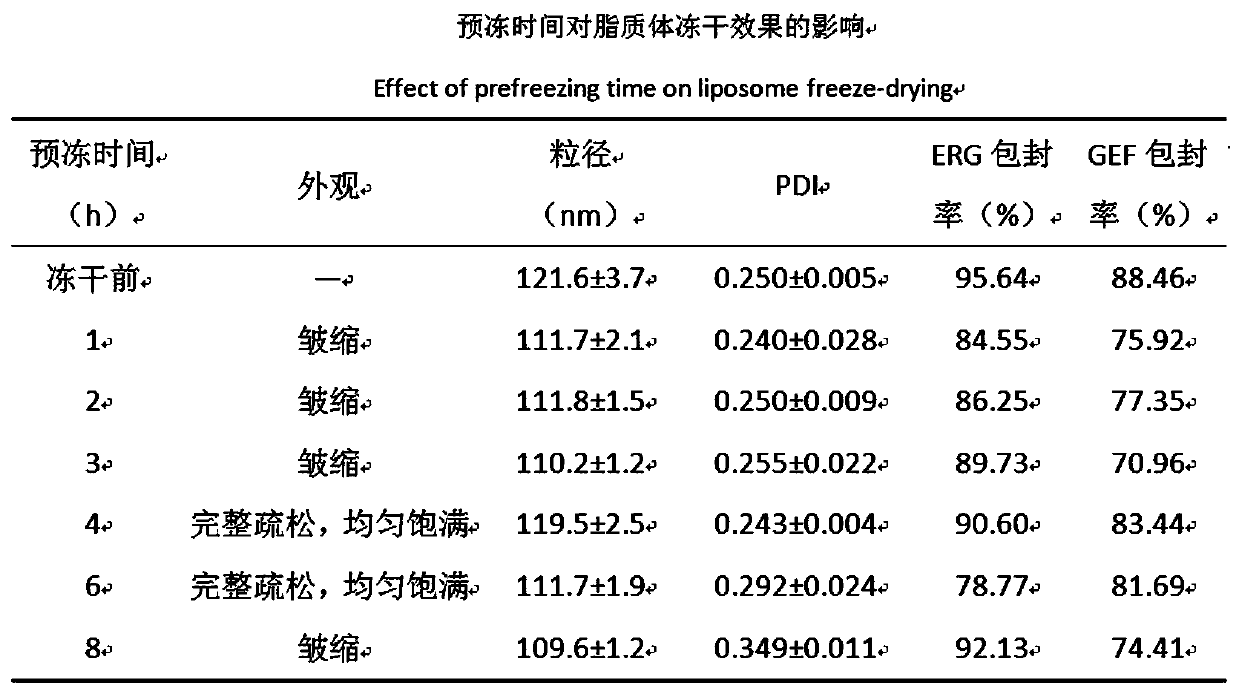
[0054] In this part, the DSPE-PEG is inserted by post-insertion method 3400 -c (RGDfk) and DSPE-PEG 1000 -R8 is embedded in the lipid membrane of ERG / GEF-LIP, and the RGD cyclic peptide / R8 peptide modified ERG / GEF-LIP (RGD / R8-ERG / GEF-LIP) is prepared. Considering the stability of the liposome itself, Prepare it in the form of freeze-dried powder, conduct preliminary investigations on its morphology, particle size d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com