Preparation and application of polymer composition loaded with sirolimus compound or its derivative
A technology of sirolimus and polymers, which is applied in the field of nano-medicine preparation, can solve problems such as disapproval of using the human body, inability to release smoothly, and increased side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] 100mg of methoxypolyethylene glycol-poly(ε-caprolactone) copolymer (MPEG 2000 -PCL 2000) and 15 mg of sirolimus were dissolved in 3 ml of absolute ethanol, the organic solvent was quickly removed under reduced pressure, and then 5 ml of preheated water for injection was added, under stirring conditions, 60 ° C into bundles, and passed through a 0.22 μm filter membrane, The filtrate was lyophilized to obtain a sample.
Embodiment 2
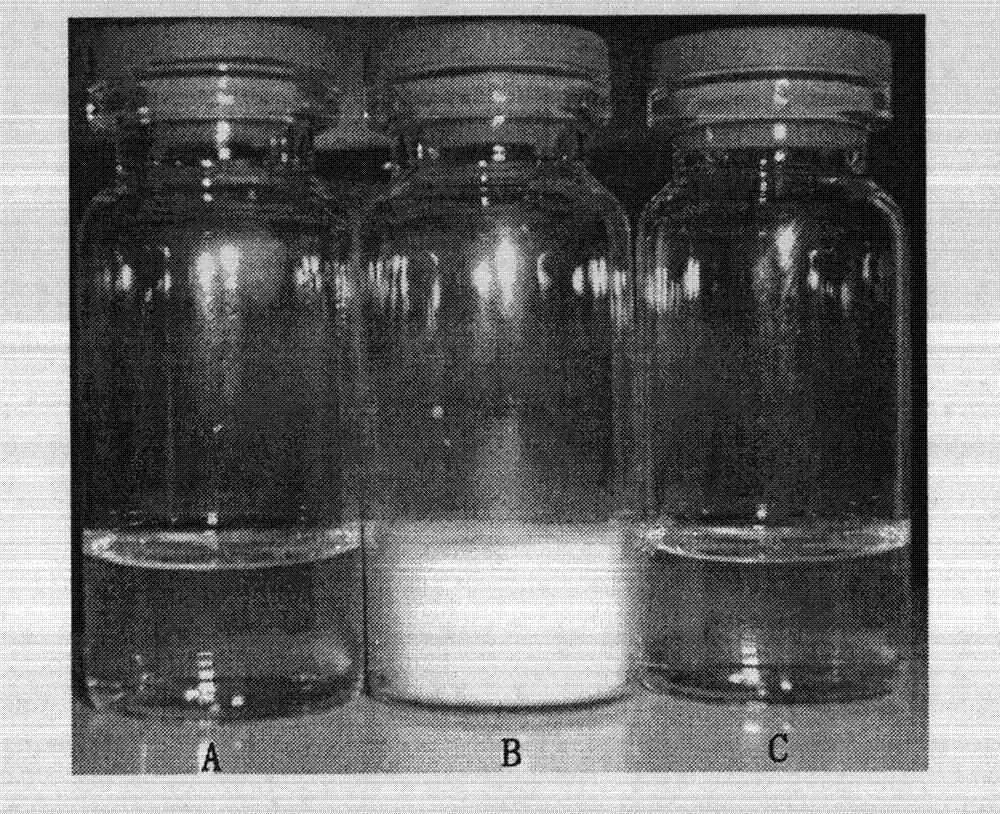
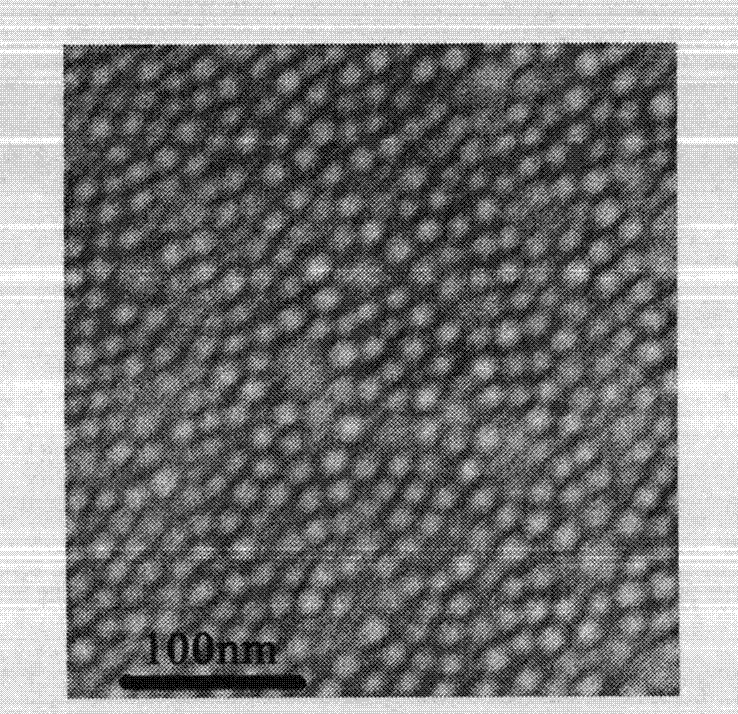
[0048] 100mg of methoxypolyethylene glycol-polylactic acid copolymer (MPEG 2000 -PLA 1700 ) and 15 mg of sirolimus were dissolved in 3 ml of absolute ethanol, the organic solvent was quickly removed under reduced pressure, and then 5 ml of preheated water for injection was added, under stirring conditions, 60 ° C into bundles, and passed through a 0.22 μm filter membrane, The filtrate was lyophilized to obtain a sample, see figure 1 , the transmission electron microscope spectrum of nanomicelle solution is shown in figure 2 .
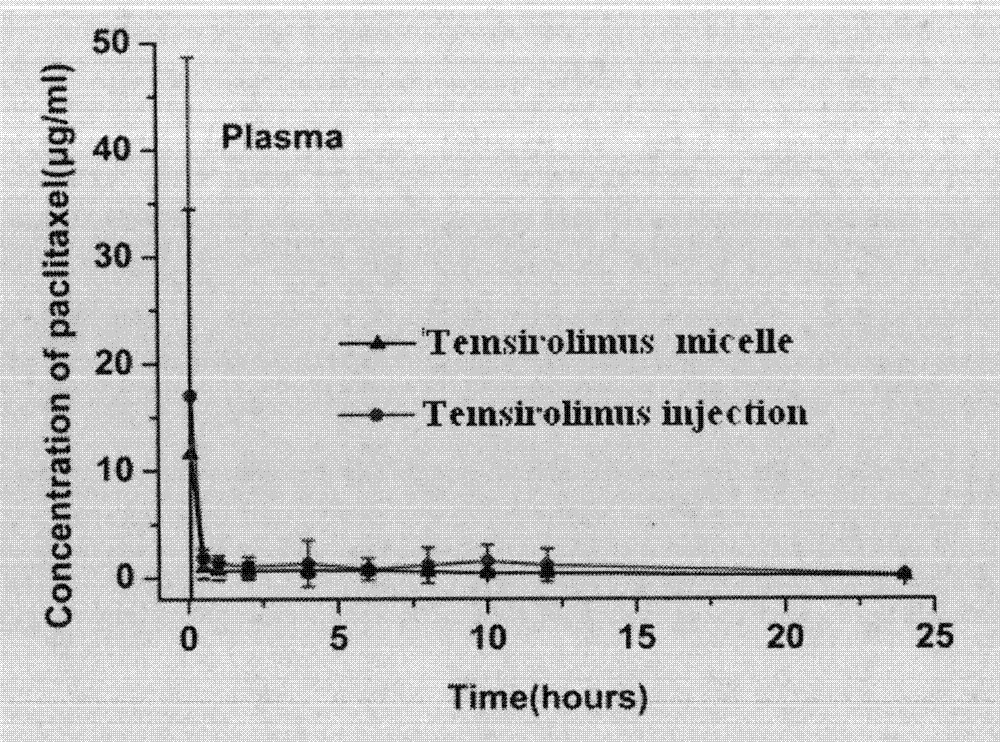
[0049] Twelve SD rats were randomly divided into two groups, 6 in each group, Temsirolimus micelle and Temsirolimus injection Sirolimus injection group. The two groups of experimental animals were injected with sirolimus nanomicelle and sirolimus injection respectively through the tail vein, and the blood was collected by docking the tail at 0.083, 0.5, 1, 2, 4, 6, 8, 10, 12, and 24 hours after administration , the blood sample was centrifuged at...
Embodiment 3
[0058] 100mg of polycaprolactone-polyethylene glycol-polycaprolactone copolymer (PCL 800 -PEG 2000 -PCL 800 ) and 15 mg of sirolimus were dissolved in 3 ml of absolute ethanol, the organic solvent was quickly removed under reduced pressure, and then 5 ml of preheated water for injection was added, under stirring conditions, 60 ° C into bundles, and passed through a 0.22 μm filter membrane, The filtrate was lyophilized to obtain a sample.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com