Strychnine gel preparation and preparation method thereof
A gel preparation, strychnine technology, applied in the directions of anti-inflammatory agents, pharmaceutical formulations, aerosol delivery, etc., can solve the problems of stratum corneum damage, high lipophilicity, skin damage, etc. Spreading, good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
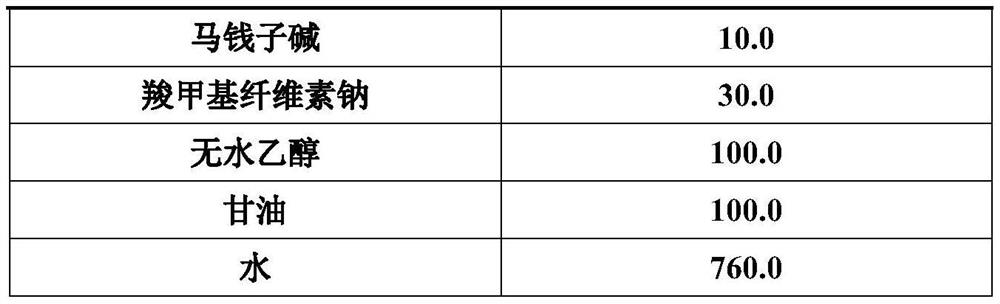
[0026] formula Dosage (g) strychnine 10.0 carbomer 8.3 Absolute ethanol 166.7 Propylene Glycol 250.0 5M NaOH 0.05 Ethylenediaminetetraacetic acid 0.83 Ethylparaben 1.67 water 1230.0
[0027] Preparation:
[0028] (1) Add 8.3g of carbomer into water to swell completely, adjust the pH value to about 6 with 5M sodium hydroxide to obtain gel matrix I; (2) dissolve 1.67g of ethylparaben in propylene glycol to obtain solution a, 0.83 g ethylenediaminetetraacetic acid is dissolved in an appropriate amount of water to obtain solution b, 10g strychnine is dissolved in absolute ethanol and solution a to obtain solution c, and solution c and b are mixed uniformly to obtain strychnine solution II; (3) condensation Gum base I is mixed with strychnine solution II and stirred evenly to obtain a strychnine gel preparation.
Embodiment 2
[0030] formula Dosage (g) strychnine 10.0 Hydroxypropylmethylcellulose 33.33 Absolute ethanol 250.0 Propylene Glycol 250.0 0.1M citric acid 0.08 Ethylenediaminetetraacetic acid 0.83 Ethylparaben 1.67 water 1120.0
[0031] Preparation:
[0032] (1) Add 33.33g of hydroxypropyl methylcellulose into water to swell completely to obtain gel matrix I; (2) dissolve 1.67g of ethylparaben in propylene glycol to obtain solution a, and dissolve 0.83g of ethylenediaminetetraacetic acid in Appropriate amount of water is obtained solution b, 10.0g strychnine is dissolved in absolute ethanol and solution a to obtain solution c, solution c and b are mixed uniformly to obtain strychnine solution II; (3) gel matrix I and strychnine Alkaline solution II was mixed, stirred evenly, and 0.1M citric acid was added to adjust the pH value to about 7 to obtain a strychnine gel preparation.
Embodiment 3
[0034] formula Dosage (g) strychnine 10.0 Hydroxypropylmethylcellulose 40.0 Absolute ethanol 200.0 Propylene Glycol 300.0 0.1M citric acid 0.10 water 1450.0
[0035] Preparation:
[0036] (1) Add 40 g of hydroxypropyl methylcellulose to water to swell completely to obtain gel matrix I; (2) dissolve 10 g of strychnine in absolute ethanol and propylene glycol, mix with the remaining prescription amount of water, and stir evenly to obtain strychnine Subalkali solution II; (3) Gel matrix I was mixed with strychnine solution II, stirred evenly, and 0.1M citric acid was added to adjust the pH value to about 7 to obtain a strychnine gel preparation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com