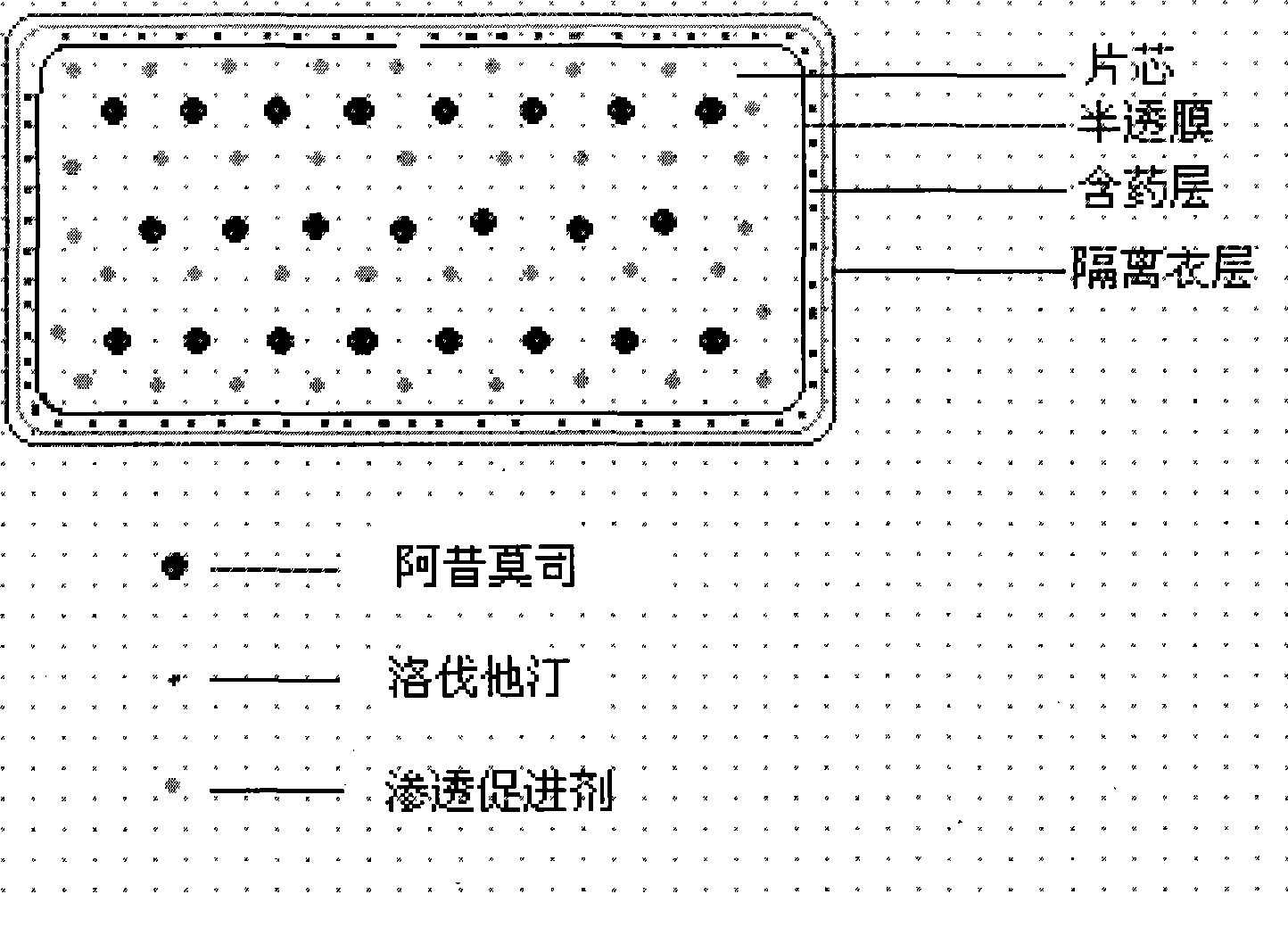
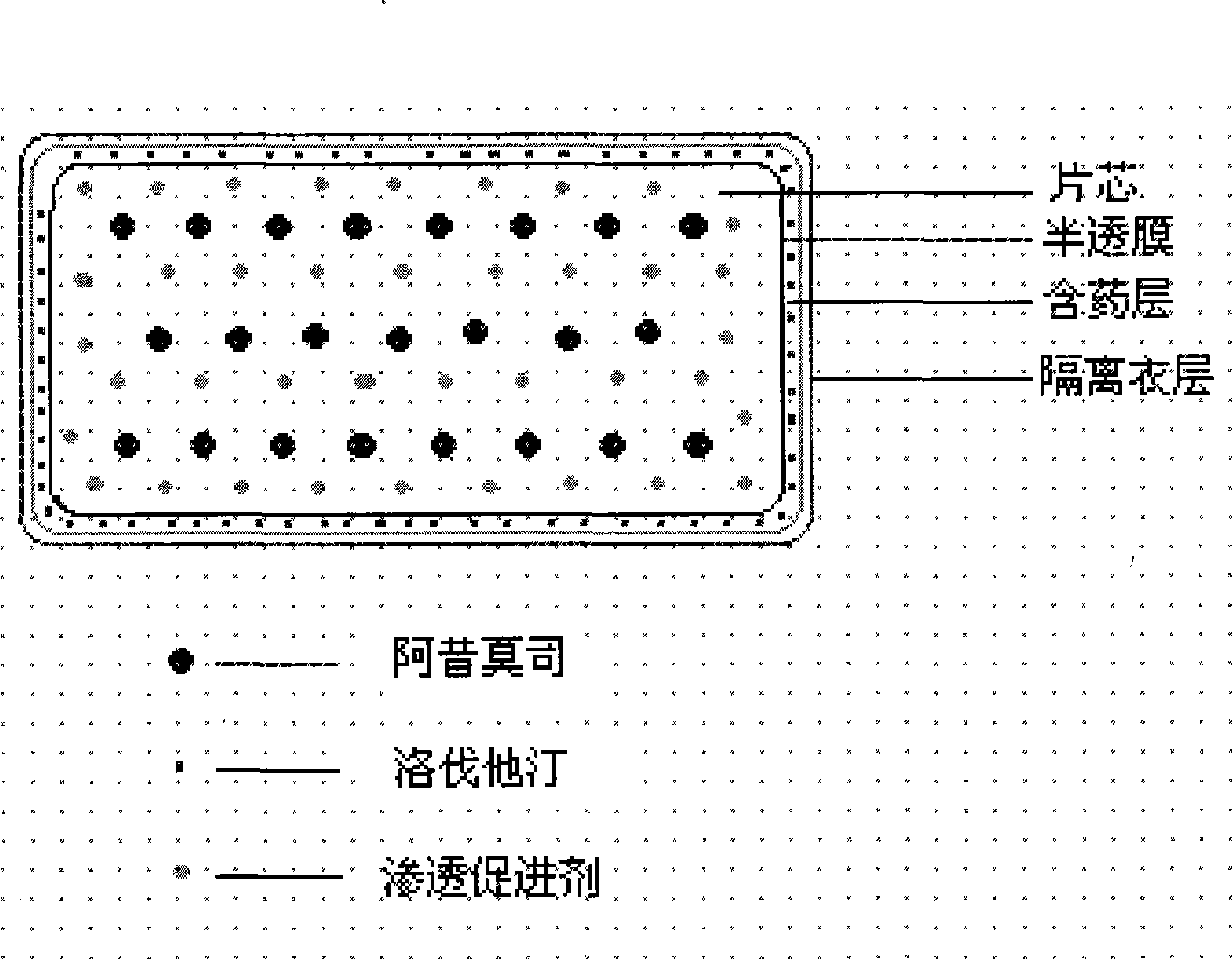
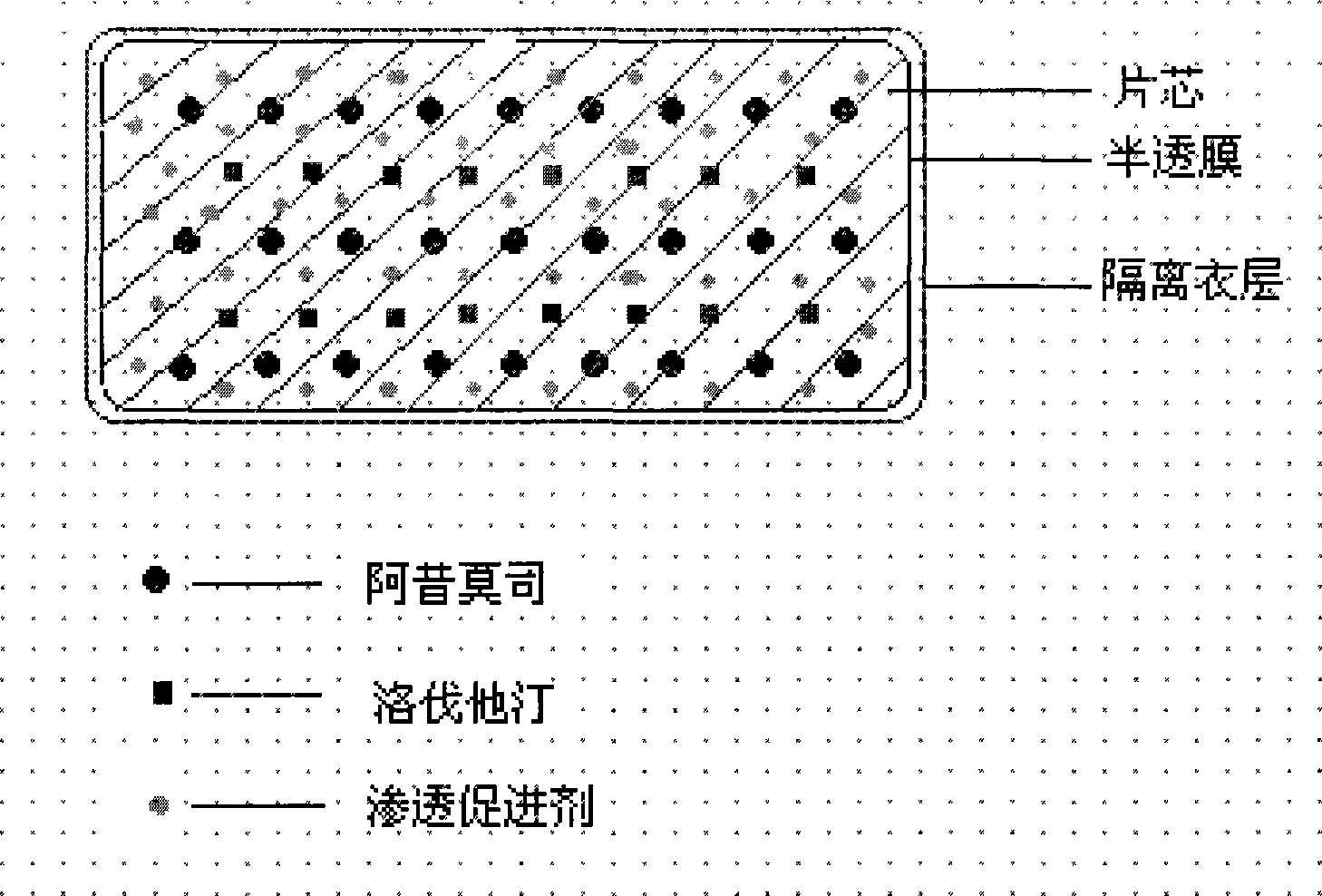
Osmotic pump controlled release preparation composition for treating hyperlipemia and preparation method thereof
A technology of osmotic pump controlled release and composition, which is applied in the field of medicine and can solve problems such as short half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Chip composition:
[0051] Acipimus 250g
[0052] NaCl 100g
[0053] PVPk30 5g
[0055] Coating film composition:
[0056] Cellulose acetate 12g
[0057] Macrogol 4000 2.5g
[0058] Diethyl phthalate 2g
[0059] Immediate release drug layer composition:
[0060] Lovastatin 5g
[0061] HPMC6cp 4g
[0063] Isolation film coat layer:
[0064] Opadry II
[0065] Make 1000 tablets by the following preparation method: (1) Tablet core preparation: get sodium chloride and pulverize, cross 100 mesh sieves, mix with acipimox evenly, with 70% ethanol solution containing 8% PVPk30 as wetting agent, Make soft material, granulate through a 20-mesh sieve, dry at 45°C for 2 hours, granulate, add magnesium stearate, mix well, and compress into tablets, using conventional tableting technology to compress 1000 tablets. (2) Tablet core coating: Take cellulose acetate, add 280ml of acetone, stir to dissolve; take another ...
Embodiment 2
[0068] Chip composition:
[0069] Chip composition:
[0070] Acipimus 250g
[0071] Fructose 50g
[0072] Lactose 50g
[0073] PVPk30 5g
[0075] Coating film composition:
[0076] Cellulose acetate 12g
[0077] Macrogol 4000 1.5g
[0078] Dibutyl sebacate 2g
[0079] Immediate release drug layer composition:
[0080] Lovastatin 7g
[0081] HPMC6cp 5g
[0082] Sodium Lauryl Sulfate 2g
[0083] Titanium dioxide 1g
[0085] Make 1000 tablets by the following preparation method: (1) Tablet core preparation: get sodium chloride and pulverize, cross 100 mesh sieves, mix with acipimox evenly, with 70% ethanol solution containing 8% PVPk30 as wetting agent, Make soft material, granulate through a 20-mesh sieve, dry at 45°C for 2 hours, granulate, add magnesium stearate, mix well, and compress into tablets, using conventional tableting technology to compress 1000 tablets. (2) Tablet core coating: Take cellulose acet...
Embodiment 3
[0088] Chip composition:
[0089] Acipimus 250g
[0090] NaCl 100g
[0091] PVPk30 5g
[0093] Coating film composition:
[0094] Ethylcellulose 12g
[0095] HPMC6cp 2g
[0096] Macrogol 4000 1g
[0097] Immediate release drug layer composition:
[0098] Lovastatin 9g
[0099] HPMC6cp 6g
[0101] Isolation film coat layer:
[0102] Opadry II
[0103] Make 1000 tablets by the following preparation method: (1) tablet core preparation Get sodium chloride and pulverize, cross 100 mesh sieves, mix with acipimox evenly, take the 50% ethanol solution containing 5% HPMC6cp as wetting agent, prepare Soft material, passed through a 20-mesh sieve to granulate, dried at 5°C for 2 hours, granulated, added with magnesium stearate, mixed evenly, compressed into tablets, and compressed into 1000 tablets by conventional tableting technology. (2) Core coating: take ethyl cellulose, add 320ml of ethanol, stir to dissolve; take p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com