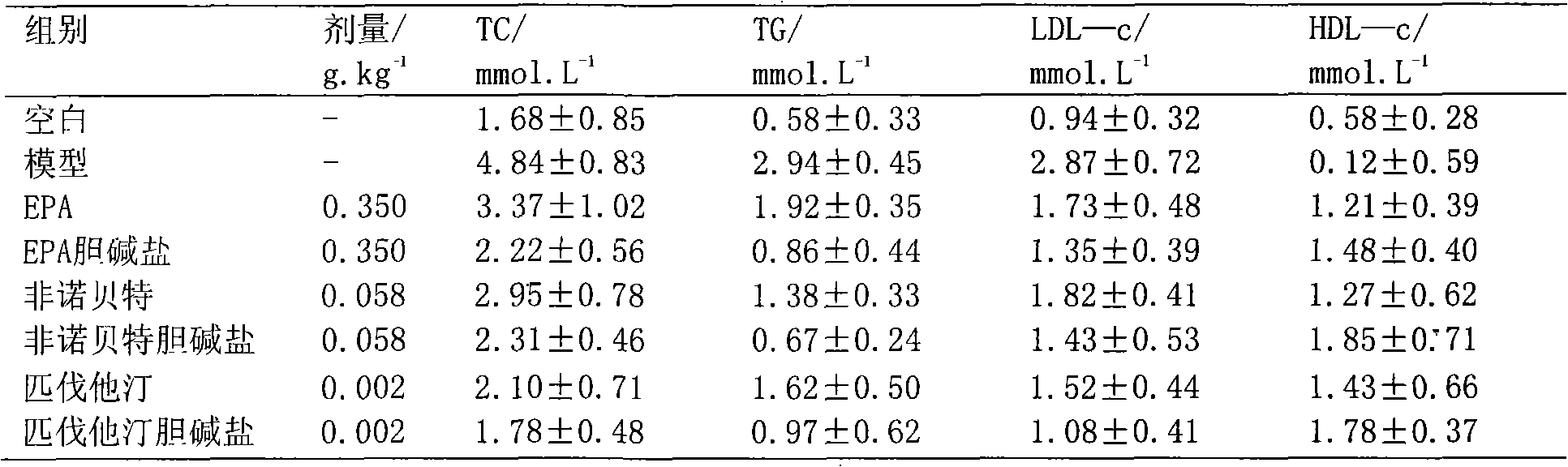
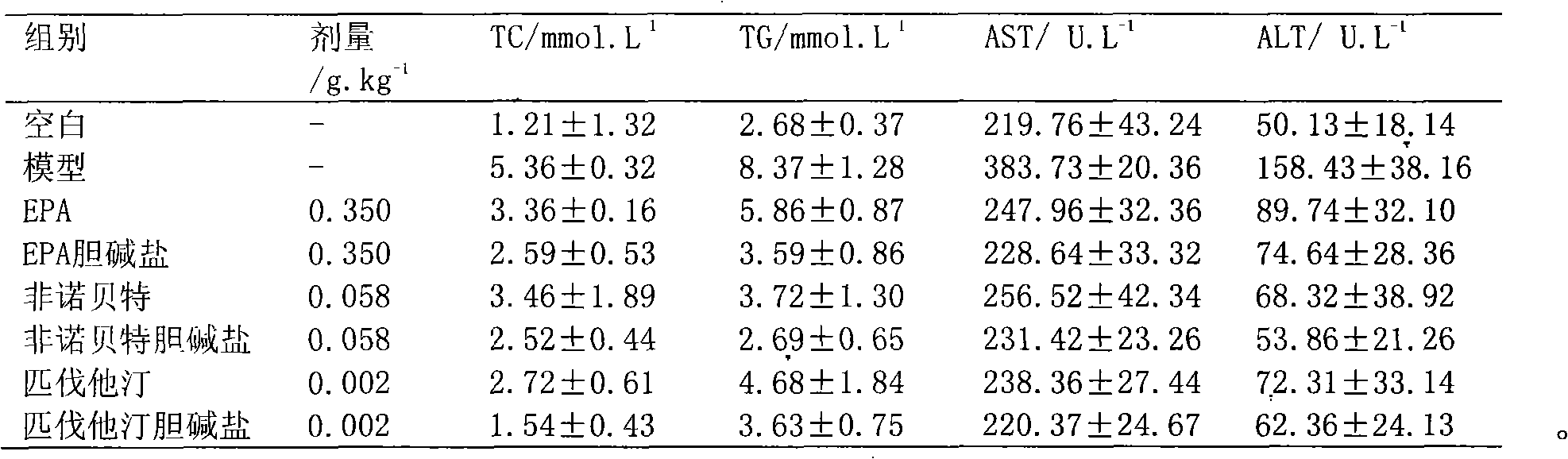
Choline salt of hypolipidemic drug and preparation method and pharmaceutical use thereof
A technology of blood lipid-lowering drugs and choline salts, which is applied in the field of medicine, can solve problems affecting bioavailability, and achieve the effects of preventing abnormal fat accumulation, improving water solubility, and enhancing drug efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] The preparation of embodiment 1 fenofibrate choline salt (I1)
[0012] Take 3.6g of fenofibrate, add 75ml of acetone to dissolve it, add 0.6g of sodium hydroxide-25ml of aqueous solution, heat and hydrolyze at 40°C, and monitor the reaction by TLC until the reaction is complete. Concentrate to remove acetone, add 25ml of water to the residue, filter, adjust the pH of the filtrate to 4 with dilute hydrochloric acid, filter, wash with water, and dry in vacuo at 45°C to obtain 2.9 g of a yellowish solid, namely fenofibric acid.
[0013] Suspend the fenofibric acid obtained above in 30ml of methanol, add 4.4g of 25% methanol solution of choline, and quickly dissolve into a light yellow transparent solution, add 60ml of ether, stir until a large amount of solids are precipitated, filter, wash with ether, After vacuum drying at 45°C, 3.2 g of white needle-like crystals were obtained. 1 H-NMR (water): 1.53 (S, 6H, N-CH3), 3.12 (S, 9H, C-CH3), 3.42-3.44 (m, 2H, CH2), 3.96-3.99...
Embodiment 2
[0014] The preparation of embodiment 2 ciprofibrate choline salt (I2)
[0015] Suspend 2.9g of ciprofibrate in 30ml of methanol, add 4.8g of 25% choline in methanol, and quickly dissolve into a light yellow transparent solution, add 60ml of diethyl ether, stir until a large amount of solids are precipitated, filter, wash with diethyl ether, 45°C After vacuum drying, 3.3 g of white solid was obtained. ESI-MS: 415.2 (M+Na + ). Elemental analysis: C (55.11%), H (6.94%), N (3.57%); calculated values: C (55.34%), H (6.82%), N (3.41%).
Embodiment 3
[0016] The preparation of embodiment 3 gemfibrozil choline salt (I3)
[0017] Referring to the method of Example 2, gemfibrozil was used instead of ciprofibrate to prepare I3 with a yield of 82%. ESI-MS: 376.7 (M+Na + ). Elemental analysis: C (67.95%), H (9.98%), N (3.96%); calculated values: C (68.22%), H (9.86%), N (3.84%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com