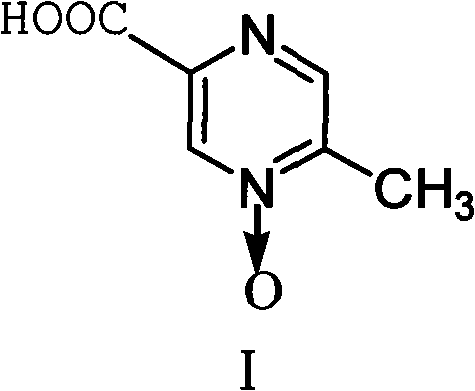
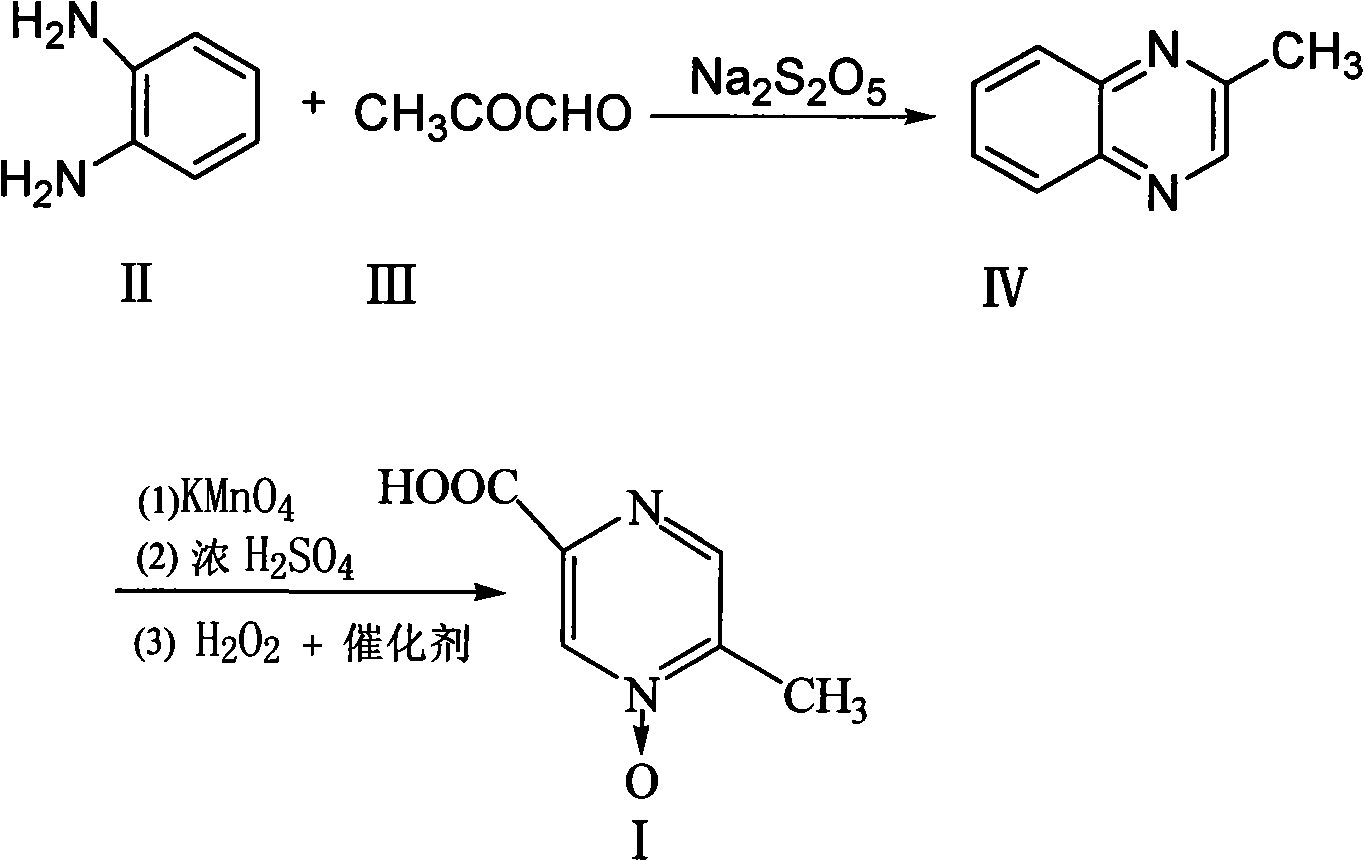
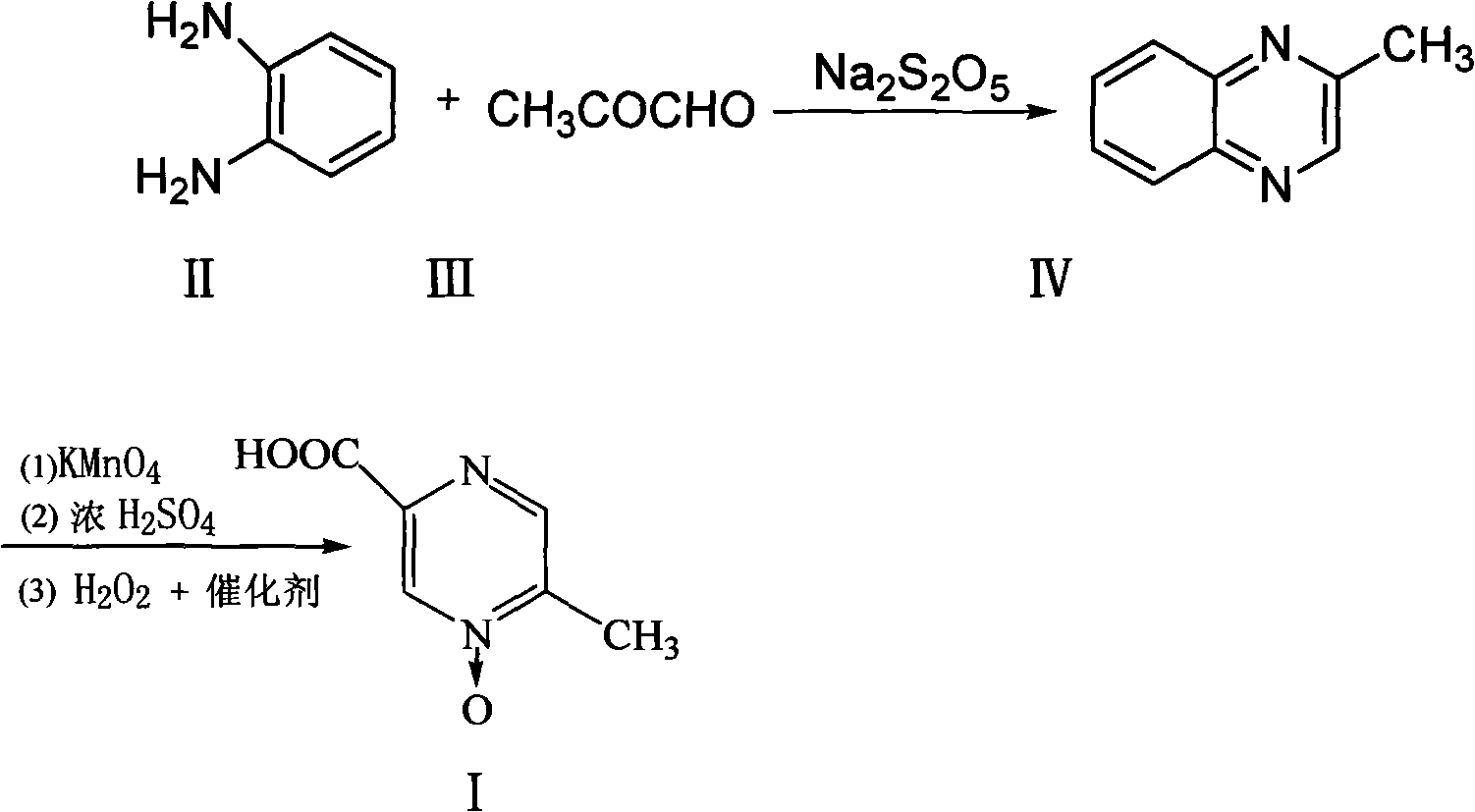
Method for improving synthesis process of Acipimox
A technology of cyclization and o-phenylenediamine, which is applied in the improvement field of acipimox synthesis process, can solve the problems of easy oxidation of intermediates by oxygen in the air, long process route, cumbersome operation, etc., to overcome easy slow oxidation, The effect of improving the reaction yield and simplifying the process steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The preparation of embodiment 1 3-methylphenprozine
[0024] Put 6.5kg of sodium metabisulfite and 13kg of water into a 100L reactor, raise the temperature to 70-80°C and stir to dissolve, add 7kg of aceguvaldehyde, add dropwise a solution of 8.5kg of o-phenylenediamine dissolved in 17kg of water at 80°C while stirring, and keep stirring to react After 35 minutes, lower the temperature to 40°C, add dropwise sodium hydroxide solution to adjust the pH to 7.0-7.5, let stand to separate the water layer, wash the organic layer twice with 50L saturated saline and pure water, and heat the fine Distillation, collecting 95 ~ 130 ° C fraction 8.2Kg, namely 3-methylbenzopyrazine, yield 72%.
Embodiment 2
[0025] The preparation of embodiment 2 3-methylbenzopyrazine
[0026] Put 7kg of sodium metabisulfite and 15kg of water into a 100L reactor, heat up to 70-80°C and stir to dissolve, add 7kg of aceguvaldehyde, add dropwise a solution of 9kg of o-phenylenediamine dissolved in 19kg of water at 80°C while stirring, and keep stirring for 60 minutes , lower the temperature to 25°C, add sodium hydroxide solution dropwise to adjust the pH to 6.8-7.0, then let stand to separate the water layer, wash the organic layer twice with 55L saturated saline and pure water, and then heat and rectify under a vacuum of 0.092MPa. Collect 8.5Kg of fractions at 95-130°C, which is 3-methylbenzopyrazine, with a yield of 75%.
Embodiment 3
[0027] Example 3 Preparation of Acipimox
[0028] Put 6kg of 3-methylbenzopyrazine into a 200L reactor, add 3kg of pure water under stirring and raise the temperature to 80°C, add dropwise a solution prepared by 38kg of potassium permanganate and 110L of water, keep it warm at 100°C to 105°C for 45 minutes, and react Complete filtration and recovery of MnO 2 , distill the filtrate to remove moisture under reduced pressure, then add 10kg of concentrated sulfuric acid dropwise and use jacket cooling water to control the temperature at 80°C, then continue to quickly add 21kg of concentrated sulfuric acid in the reaction kettle, the temperature is controlled at 105~110°C, and keep warm after adding After reacting for 1 hour, cool to 45°C, add sodium tungstate aqueous solution (Na 2 WO 4 2H 2 O260g, H 2 (013.2L) and 3.2L of 30% hydrogen peroxide were reacted at 70°C for 3.5 hours, the reaction solution was cooled, filtered with suction, and dried to obtain 5.8kg of acipimox wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com