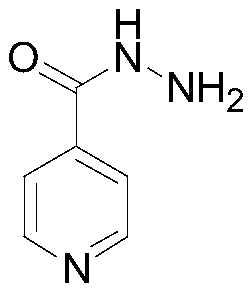
Preparation method of isoniazid
A technology of isoniazid and isoniazid, applied in the direction of organic chemistry, etc., can solve the problems of incomplete reaction of isoniazid, viscous system, low purity of isoniazid, etc., and shorten the reaction time and reaction conditions of hydrazine condensate hydrolysis. Mild, high-efficiency effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] This embodiment provides a method for preparing isoniazid, which mainly includes the following steps:
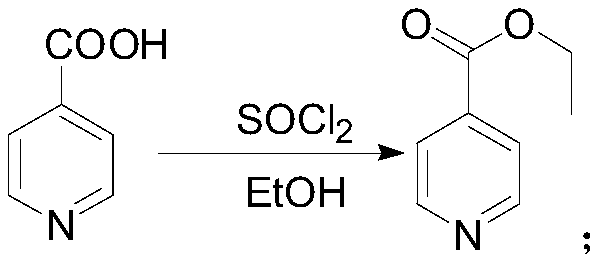
[0035] 1) Using isonicotinic acid, alcohol and an acylating agent as raw materials for esterification to obtain isonicotinate;
[0036] 2) drop isonicotinate into an alcohol solution of hydrogen chloride in an ether reagent, and react to obtain isonicotinate hydrochloride;
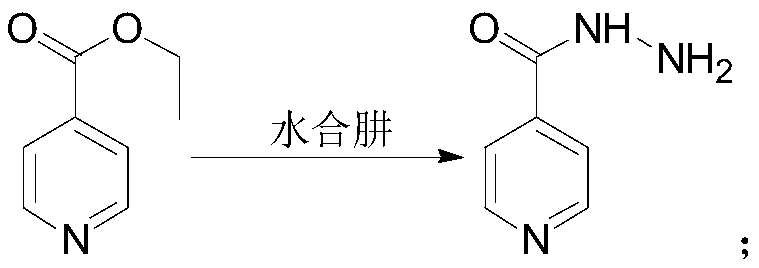
[0037] 3) After freeing isoniazid ester hydrochloride, carry out hydrazinolysis reaction with hydrazine hydrate to obtain crude isoniazid;
[0038] 4) After refining the crude product of isoniazid, the finished product is obtained.
[0039] This embodiment provides a new synthetic route for isoniazid, in view of the existence of insufficiently reacted isonicotinic acid, isonicotinamide introduced from isonicotinic acid raw materials and potential 2 - picolinic acid impurity, and both isonicotinic acid and 2-pyridinecarboxylic acid can transfer reaction, cause the purity of the isoniazid that mak...
Embodiment 1
[0055] The preparation of embodiment 1 ethyl isonicotinate hydrochloride
[0056] Add 200g (1.62mol) of isonicotinic acid, 1600ml of dichloromethane, and 10ml of N,N-dimethylformamide into a 3000ml four-necked bottle to obtain a white suspension. Slowly add 620g (4.86mol) of oxalyl chloride dropwise. After the dropwise addition is completed, stir at a temperature of 10-50°C for 4 hours, then add 150.4g (3.24mol) of absolute ethanol dropwise, and stir for 30 minutes after the dropwise addition. The aqueous sodium carbonate solution prepared in advance (220g sodium carbonate dissolved in 800ml water) was adjusted to pH=7-8, separated, and the lower organic phase was washed with 600g 15% sodium chloride aqueous solution. Concentrate the organic phase under reduced pressure at 45°C until there is no obvious fraction, add 400ml of methyl tert-butyl ether, concentrate under reduced pressure at 50°C until there is no obvious fraction, add 3200ml of methyl tert-butyl ether to the conc...
Embodiment 2
[0057] The preparation of embodiment 2 isoniazid crude products
[0058] Add 250 g (1.33 mol) of isonicotinic acid ethyl ester hydrochloride prepared in Example 1 into a 2000 ml three-necked bottle, 250 ml of purified water, stir to dissolve, and add dropwise the sodium carbonate aqueous solution prepared in advance (42.5 g sodium carbonate dissolved in 500 ml water) to pH=7~8, then add 250ml of dichloromethane, separate the layers, collect the lower organic phase, add 300ml of purified water, drop in 116.5g (1.87mol) of 80% hydrazine hydrate, heat up to 60~100°C, and Separation of dichloromethane and other low-boiling fractions while heating up, heat at 60-100°C for 0.5-4 hours, after the reaction is complete, cool down to 0-5°C, keep warm for 1 hour, filter with suction, rinse with 250ml of absolute ethanol to get a white solid powder, Air-dried at 50°C for 3 hours to obtain 148.9 g of crude isoniazid, with a yield of 81.6% and a purity of 99.92%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com