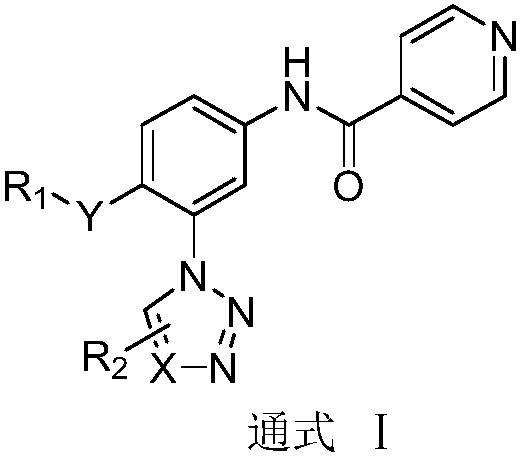
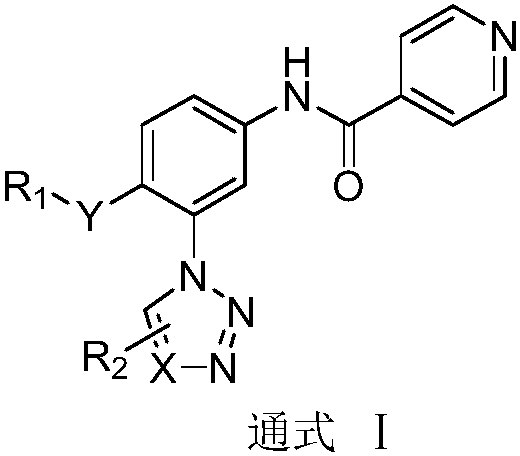
N-(3-azolylphenyl) isonicotinamide compounds as well as preparation method and application thereof
A technology of azolyl phenyl and isonicotinamide is applied in the field of medicine to achieve the effects of high yield, improved activity and easy mass production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Preparation of 4-nitro-2-(1H-tetrazol-1-yl)phenol
[0061] Add 2-amino-4-nitrophenol (10.0g, 64.88mmol), glacial acetic acid (100mL) and triethyl orthoformate (20.7g, 129.67mmol) into a 500mL reaction flask, slowly add sodium azide under stirring (8.4g, 129.67mmol), react overnight at 80°C. Cool after the reaction, pour into ice water, filter with suction, wash the filter cake with plenty of water, and dry naturally to obtain 11.7 g of reddish-brown solid, yield: 87.1%.
Embodiment 2
[0063] Preparation of 4-amino-2-(1H-tetrazol-1-yl)phenol
[0064] Add 4-nitro-2-(1H-tetrazol-1-yl)phenol (6.0g, 28.96mmol), reduced iron powder (6.5g, 115.84mmol), ammonium chloride (3.1g ,57.92mmol), ethanol (90mL) and water (30mL), reflux at 80°C overnight, the reaction was complete, diluted with water, extracted with ethyl acetate (50mL*3), washed with saturated brine, dried over anhydrous sodium sulfate, filtered, Concentrate under reduced pressure to dryness to obtain 4.24 g of refined product, yield: 82.6%.
Embodiment 3
[0066] Preparation of 4-isonicotinamide-2-(1H-tetrazol-1-yl)phenylisonicotinic acid methyl ester
[0067] Add 4-amino-2-(1H-tetrazol-1-yl)phenol (1.0g, 5.64mmol), triethylamine (5.2g, 50.76mmol) and dichloromethane (20mL) in 250mL reaction flask,- Add dichloromethane (20mL) and isonicotinic acid chloride (4.02g, 22.56mmol) dropwise under stirring at 5°C. After maintaining the temperature for 1 hour, add water, separate the organic layer, extract the aqueous layer with dichloromethane, and combine the organic layers. Wash with saturated brine, dry over anhydrous sodium sulfate, filter, and concentrate under reduced pressure to dryness to obtain 1.74 g of refined product, yield: 79.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com