Application of HPMC and naringenin isonicotinamide co-crystal in preparation of medicine for preventing and treating abdominal aortic aneurysm
An abdominal aortic aneurysm and isonicotinamide technology, applied in the field of medicine, can solve the problems of increasing absorption and drug efficacy, not increasing naringenin supersaturation, and not being clear
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1、1
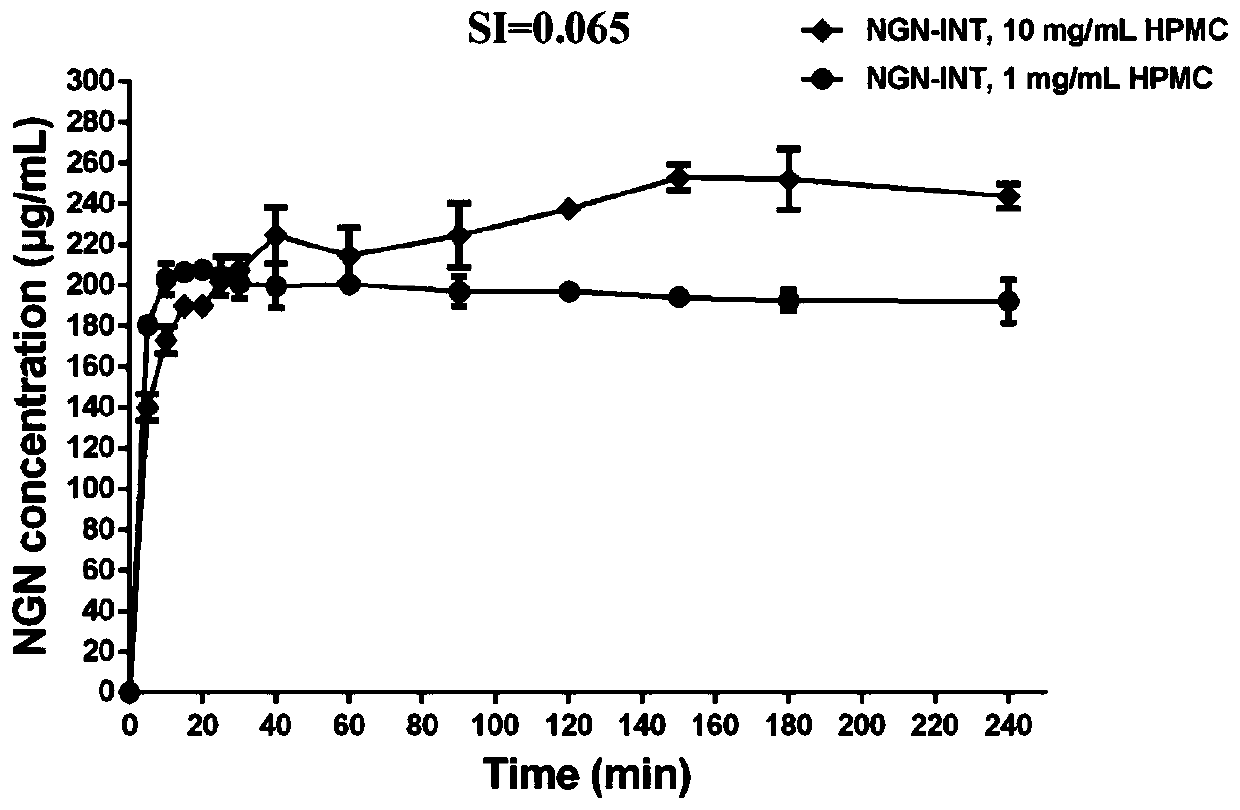
[0031] Embodiment 1, the influence of 1mg / mL HPMC on NGN-INT
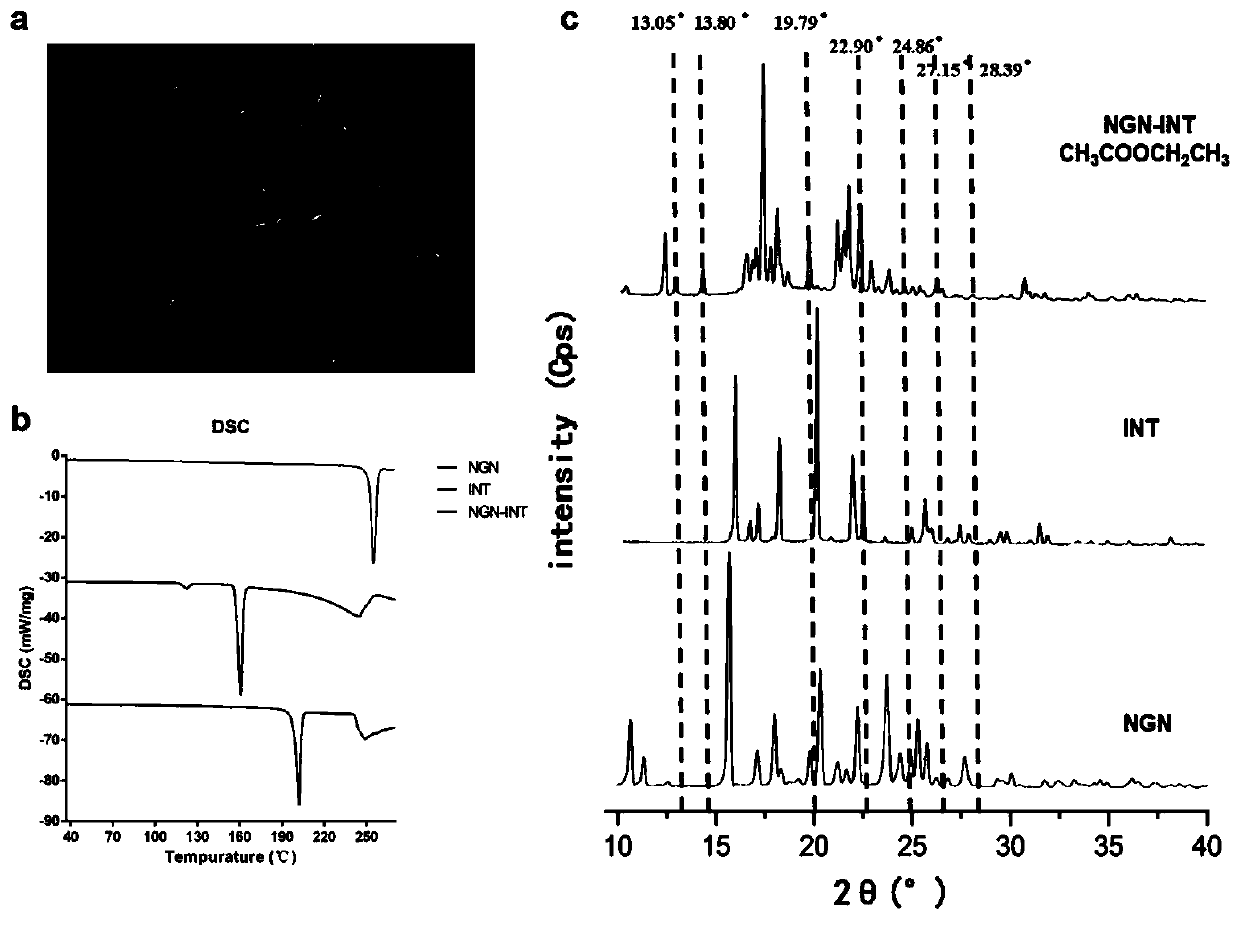
[0032] Accurately weigh 252.2 mg of NGN and 226.2 mg of INT into 50 mL of ethyl acetate solution and stir until all solids are completely dissolved. The solution was stirred for an additional 12 hours until the ethyl acetate solution had completely evaporated at room temperature. The obtained crystals were vacuum-dried overnight, and white crystals of NGN-INT were collected. The characterization results are shown in figure 1 .
[0033] The prepared NGN-INT is passed through a 80-100 mesh sieve, so that the particle size of the powder is 165-200 μm. NGN-INT (equivalent to 75 mg NGN) was dissolved in 125 mL of dissolution medium (50 mM pH 6.8 PBS buffer containing 1 mg / mL HPMC) (SI=0.065) with magnetic stirring at room temperature. At 5, 10, 15, 20, 25, 30, 40, 60, 90, 120, 150, 180 and 240min, take out 1mL of solution from the medium, centrifuge the taken out solution (12000rpm, 3min), take the upper layer soluti...
Embodiment 2、10
[0034] Embodiment 2, the influence of 10mg / mL HPMC on NGN-INT
[0035] NGN-INT was prepared according to the method described in Example 1, and passed through a sieve of 80-100 mesh, so that the particle size of NGN-INT was 165-200 μm.
[0036]NGN-INT (equivalent to 75 mg NGN) was dissolved in 125 mL of dissolution medium (50 mM pH 6.8 PBS buffer containing 10 mg / mL HPMC) (SI=0.065) with magnetic stirring at room temperature. At 5, 10, 15, 20, 25, 30, 40, 60, 90, 120, 150, 180, and 240 min, take out 1 mL of solution from the medium, centrifuge the taken out solution (12000 rpm, 3 min), and take the upper layer solution. After passing through a 0.45 μm polyethersulfone filter membrane, the UV absorption of NGN at each time point was detected by UV spectrophotometry, and converted to the content of NGN in the dissolution medium, and the cumulative release was taken as the ordinate and time as the abscissa to draw the dissolution curve ( see the results figure 2 ).
[0037] D...
Embodiment 3
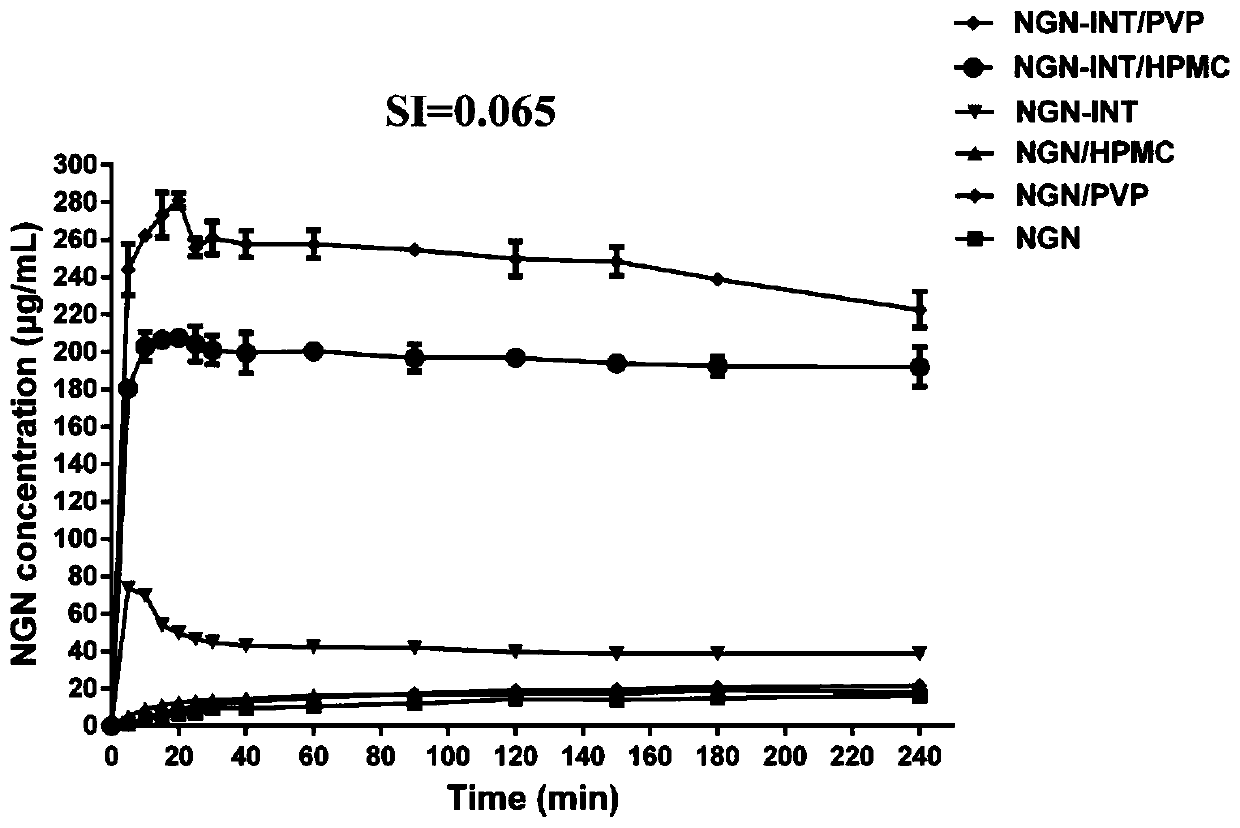
[0038] Example 3. HPMC / PVP promotes the release of NGN in NGN-INT
[0039] Sun[1] et al proposed the concept of sink-index to describe the dissolution conditions, and SI is used to represent the dissolution conditions below. Three parts of 75.0 mg NGN and three parts of NGN-INT (equivalent to 75.0 mg NGN) prepared in Example 1 above were passed through a 80-100 mesh sieve to make the particle size of the powder 165-200 μm. One part NGN and one part NGN-INT were dissolved in 125mL of 50mM PBS buffer, pH 6.8; one part NGN and one part NGN-INT were dissolved in 125mL of dissolution medium (50mM pH 6.8 PBS buffer containing 1mg / mL PVP). solution); one portion of NGN and another portion of NGN-INT were dissolved in 125 mL of dissolution medium (50 mM pH 6.8 PBS buffer containing 1 mg / mL HPMC) (SI=0.065) with magnetic stirring at room temperature. At 5, 10, 15, 20, 25, 30, 40, 60, 90, 120, 150, 180 and 240min, take out 1mL of solution from the medium, centrifuge the taken out solut...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com