Genetic engineering strain taking tyrosine as substrate to synthesize naringenin and construction method thereof
A technology of genetically engineered bacteria and tyrosine, applied in the field of synthetic biology or metabolic engineering, can solve the problems of low solubility and high price of coumaric acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The selection of embodiment 1 pathway gene
[0030] Phenylalanine deaminase (TAL) widely exists in higher plants, fungi, yeast and a kind of prokaryotic streptomyces. Human beings have not found this enzyme in eubacteria or animal tissues so far. Although TALs are widely distributed, only R. glutinis is used commercially, and R. glutinis has a higher catalytic activity for tyrosine than other species. In recent studies, 4-coumaric acid: Coenzyme A ligase (4CL) from P. hybrida, chalcone synthase (CHS) from P. hybrida, Combination of chalcone isomerase (CHI) to obtain various unnatural flavonoid skeleton substances. Therefore, the present invention selects TAL of Rhodotorula glutinis, 4CL of parsley (Petroselinum crispum), CHS of petunia (Petunia X hybrida), and CHI (M91079) of alfalfa (Medicago sativa).
[0031]The development of natural product production platforms is usually limited by precursor substances or cofactors, because the amount of these substances in the h...
Embodiment 2
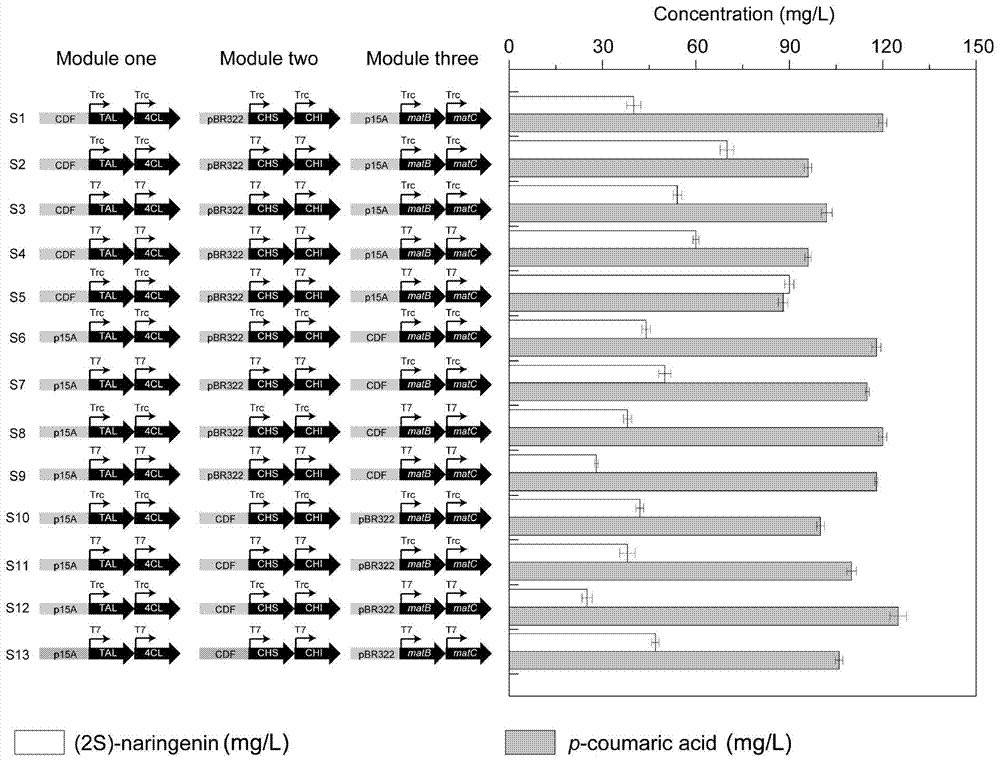
[0033] Example 2 Synthetic route optimization based on modular transformation theory
[0034] For the constructed synthetic pathway, this study intends to divide it into three modules, mainly based on the following principles: i) The product of 4CL, coumaroyl-CoA / cinnamoyl-CoA, will inhibit the TAL gene in the pathway, so TAL and 4CL is divided into one module, and CHS and CHI are divided into one module. It is intended to alleviate this feedback inhibition by strengthening the downstream module and weakening the upstream module; Low content usually limits the production of flavonoids in bacteria, so the malonyl-CoA synthesis pathway (matB and matC) is placed in a separate module, and the optimal malonyl-CoA content is found by changing the metabolic flux of this module . Therefore, the total approach is divided into three modules: module one is composed of TAL and 4CL; module two is composed of CHS and CHI; module three is composed of matB and matC. The metabolic flux of ea...
Embodiment 3
[0036] Embodiment 3 contains the construction of optimized module engineering bacteria
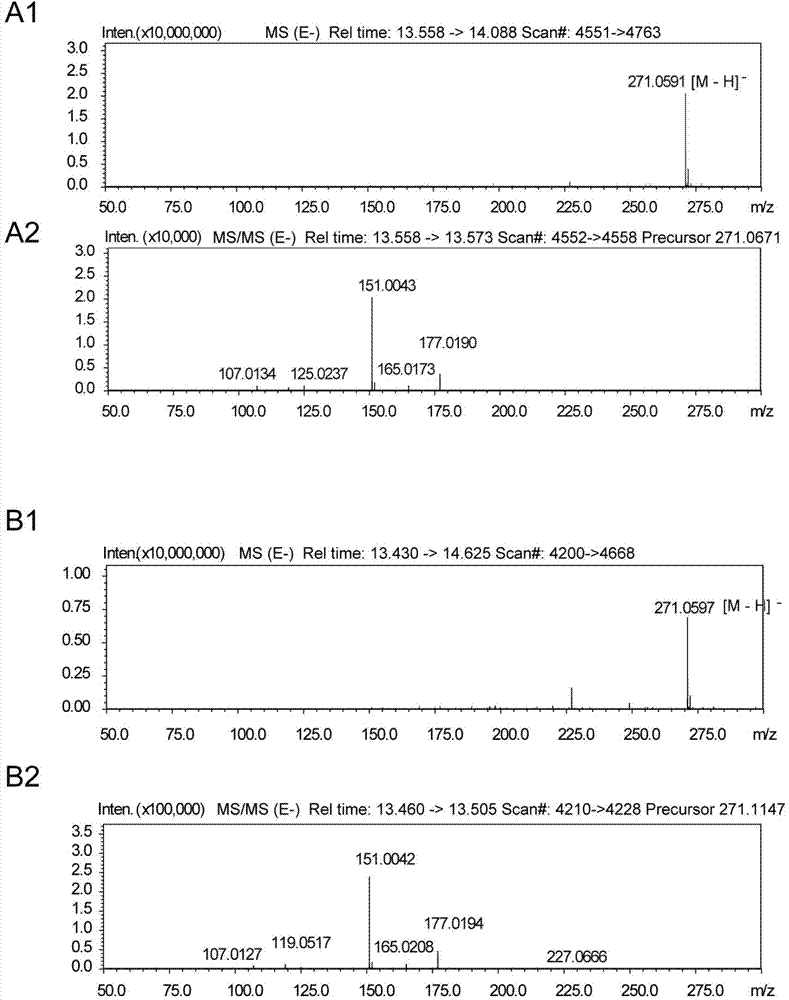
[0037] The six genes needed for the pathway were obtained by using the whole gene synthesis method, and the sizes were 2.1Kb, 1.7Kb, 1.5Kb, 0.9Kb, 1.5Kb, and 1.3Kb, respectively. Ligate the TAL, 4CL genes with the plasmids digested with NcoI and HindIII, NdeI and BlnI respectively, connect the CHS and CHI genes with the plasmids digested with NcoI and HindIII, NdeI and BlnI, and connect the matB The matC gene was connected with the plasmid digested with EcoRI, HindIII, NdeI and KpnI, thus obtaining three co-expression plasmids pCDF-RgTAL-Pc4CL, pET-PhCHS-MsCHI, and pACYCD-matB-matC. The constructed recombinant plasmid was digested and analyzed, and DNA sequencing was carried out. The results of gene sequencing were consistent with expectations, indicating that the recombinant plasmid was constructed correctly. The three co-expression plasmids were chemically transformed into Escherichia ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com