Method for preparing scutellarin and analogues thereof
A technology for scutellarin and its analogues, applied in the field of preparation of scutellarin and its analogues, achieving good bioavailability and flexible reaction routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0022] The first aspect of the present invention is to provide a method for synthesizing scutellarin.
[0023] The method for synthesizing scutellarin of the present invention comprises:
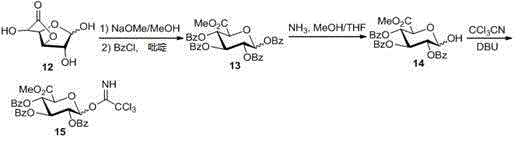
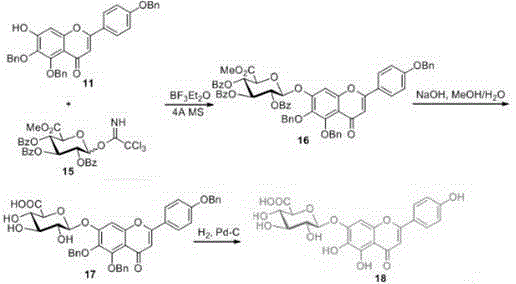
[0024] The process of synthesizing 7-OH acceptor compound with naringenin as raw material; the process of synthesizing glucuronic acid donor compound with glucuronic acid 3,6 lactone as raw material; and the acceptor compound and donor compound obtained from the aforementioned process Synthesis of the final product scutellarin.
[0025] The specific synthesis steps are described below.
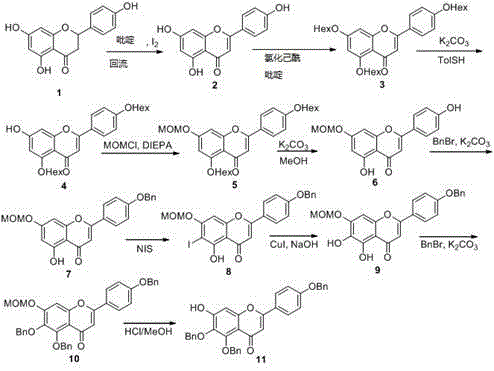
[0026] Synthesis of 7-OH acceptor compound 11
[0027] The following reaction scheme (A) shows in detail the specific steps for synthesizing the 7-OH acceptor compound represented by structural formula 11 using naringenin represented by structural formula 1 as a raw material.
[0028] Under the action of a catalytic amount of iodine, in the presence of pyridine and under reflux conditions, naringenin 1 u...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com