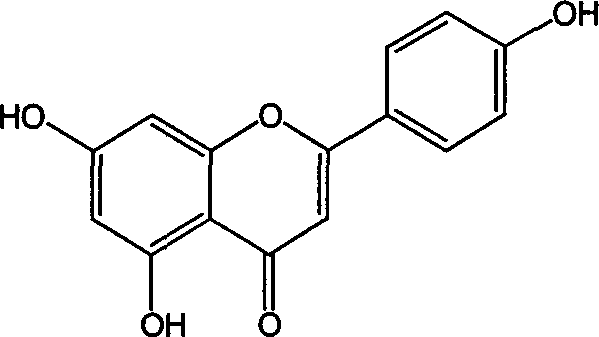
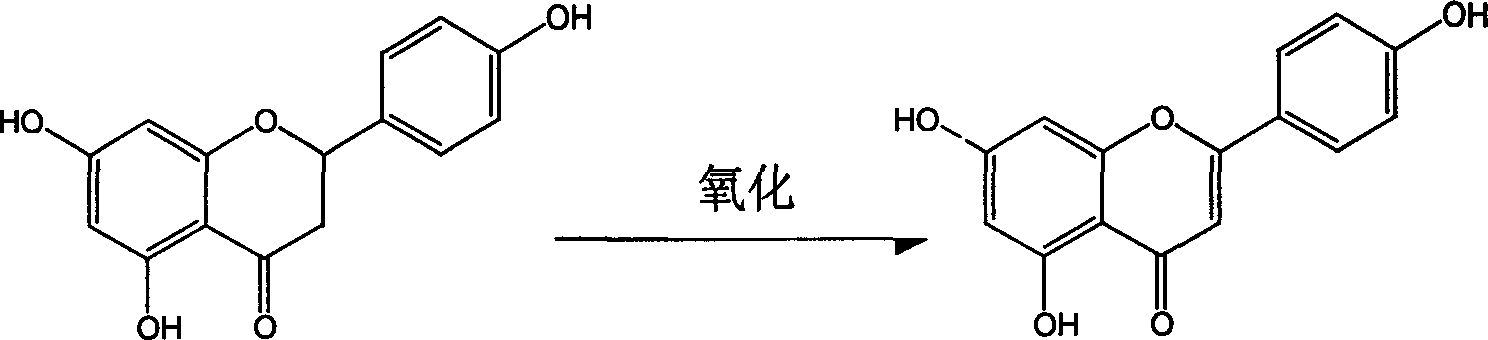
Process for semi-synthesizing of apiolin
A synthesis method and technology of apigenin, applied in directions such as organic chemistry, can solve problems such as unfriendly odor to human body, increase production cost, unfavorable industrial production, etc., and achieve the effects of easy control of reaction conditions, easy control, and reduction of synthesis cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Example 1 40g naringenin, 0.5g sodium hydroxide and 30.48g iodine were dissolved in 400ml 1,4-dioxane and refluxed for 6 hours (120°C). Concentrate the reaction solution to 100ml, add 700ml of water, let stand, and filter with suction. The obtained crude product apigenin was fully dissolved with 600ml of 5% NaOH solution, then methanol was added, and the pH value was adjusted to 6 with hydrochloric acid, a large amount of crystals were precipitated, and then suction filtered. The apigenin obtained above was purified by reflux with 150 ml of acetone, filtered and dried to obtain 24 g of apigenin with a purity of 98.2%, and a yield of 60%.
Embodiment 2
[0017] Dissolve 40g of naringenin, 0.7g of potassium hydroxide and 38.10g of iodine in 500ml of 1,4-dioxane, and react in a constant temperature water bath at 100°C for 8 hours. Concentrate the reaction solution to 120ml, add 700ml of water, let stand, and filter with suction. The obtained crude product apigenin was fully dissolved with 500ml of 10% KOH solution, then 800ml of glycerin was added, and the pH value was adjusted to about 5 with hydrochloric acid, a large amount of crystals were precipitated, and then suction filtered. Wash until neutral, dry. Dissolve with 150ml dimethylformamide, add water, and precipitate. After drying, 28 g of apigenin with a purity of 98.5% were obtained, and the yield was 65.4%.
Embodiment 3
[0019] Dissolve 40g of naringenin, 0.5g of potassium hydroxide and 38.10g of iodine in 300ml of 1,4-dioxane in a constant temperature water bath of 50°C. Concentrate the reaction solution to 100ml, add 600ml of water, let stand, and filter with suction. The obtained crude product apigenin was fully dissolved with 800ml of 3% NaOH solution, then 800ml of ethanol was added, and the pH value was adjusted to 6 with hydrochloric acid, a large amount of crystals were precipitated, and then suction filtered. Wash until neutral, dry. Dissolve with 150ml dimethyl sulfoxide, filter, add water, and precipitate. Obtain 20g apigenin, its purity is 97.8%, yield 50%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com