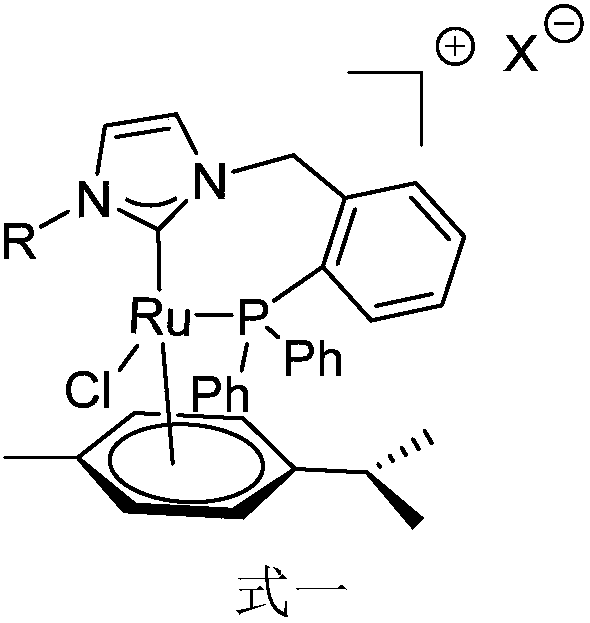
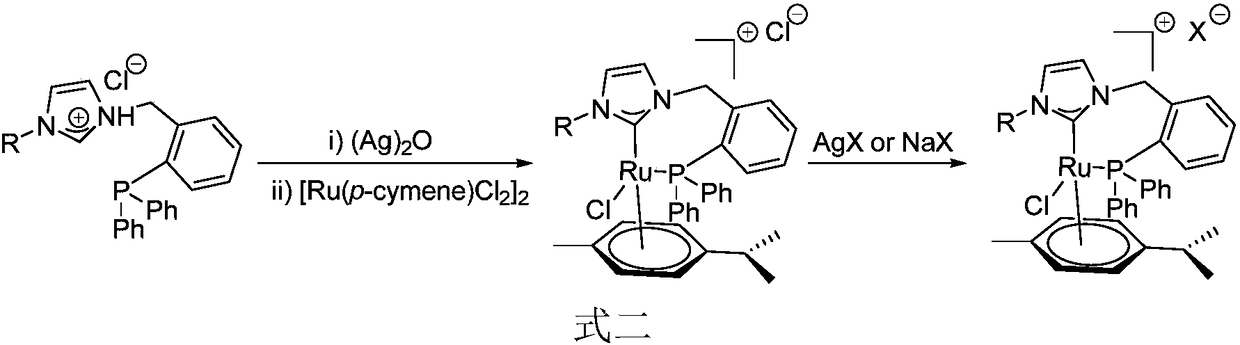
Novel bidentate phosphorus-N-heterocyclic carbine p-cymene type ruthenium complex catalyst as well as preparation method and synthetic application thereof
An azacarbene and p-cymene technology, which is applied in the field of a novel bidentate phosphorus-azacarbene p-cymene-type ruthenium complex catalyst and its preparation, can solve problems such as hindering industrial applications, and achieve simple and easy operation steps The effect of running, air stability, and adaptability to a wide range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
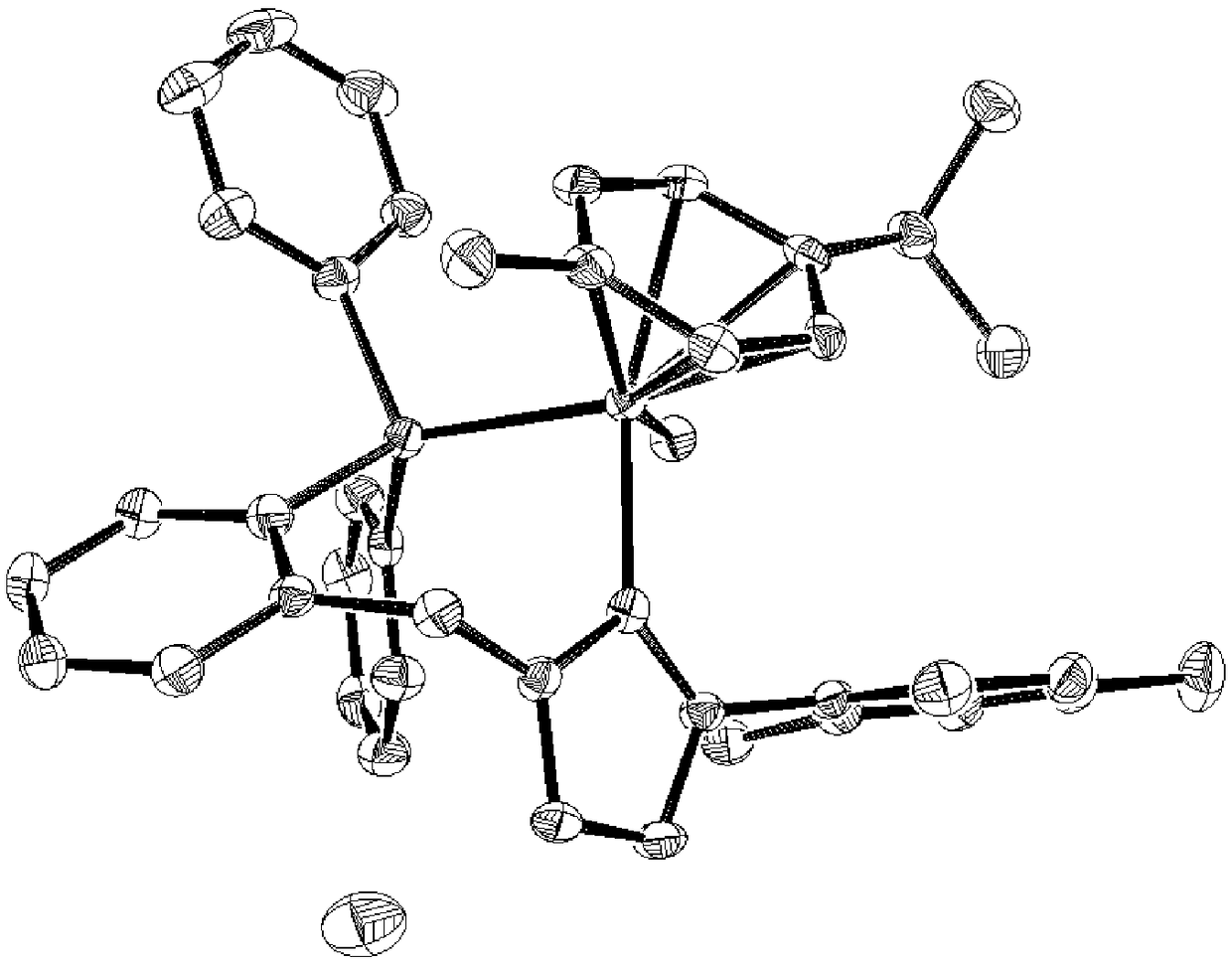
Image
Examples
Embodiment 1
[0052] Synthesis of the bidentate phosphorus-azaimidazole chloride salt ligand 5a was carried out in accordance with the following steps: 316mg (1.02mmol) o-diphenylphosphine benzyl chloride and 186mg (1.0mmol) 1-metrimethylphenylimidazole were dissolved in 2ml of anhydrous DMF was stirred at 90°C for 48h under the protection of argon. Cool to room temperature, spin dry the solvent, and recrystallize the crude product from dichloromethane-ethyl acetate to obtain 367 mg of white solid, bidentate phosphorus-azimidazolium chloride ligand 5a, with a yield of 74%.
[0053] 1 H NMR (400MHz, DMSO-d 6 )δ9.84(s,1H),7.97(d,J=6.9Hz,2H),7.58–7.49(m,2H),7.42(s,7H),7.24–7.21(m,4H),7.12(s ,2H), 7.00–6.97(m,1H), 5.79(s,2H), 2.31(s,3H), 1.97(s,6H).
[0054] 13 C NMR (101MHz, DMSO-d 6)δ140.1, 138.2, 138.2, 137.9, 136.2, 136.1, 135.0, 134.9, 134.2, 134.1, 133.4, 133.2, 131.1, 130.2, 130.0, 130.0, 129.6, 129.3, 129.2, 129.0, 124. , J=24.2Hz), 20.6, 16.9.
[0055] 31 P NMR (162MHz, DMSO-d ...
Embodiment 2
[0058] Synthesis of bidentate phosphorus-azimidazole chloride salt ligand 5b, specifically according to the following steps:
[0059] According to the synthesis method of ligand 5a in Example 1, 1-me-trimethylphenylimidazole was replaced with 144mg (1.0mmol) 1-phenylimidazole, and other operating conditions were the same as in Example 1. After the reaction, 390mg of white solid was obtained. Namely, the bidentate phosphorus-azaimidazole chloride ligand 5b, and its yield was 86%.
[0060] 1 H NMR (400MHz, DMSO-d 6 )δ9.91(s,1H),8.17(s,1H),7.78(s,1H),7.73–7.66(m,1H),7.66–7.59(m,4H),7.56(t,J=7.1Hz , 2H), 7.46(t, J=7.4Hz, 1H), 7.33(s, 6H), 7.18–7.04(m, 4H), 6.95–6.92(m, 1H), 5.78(s, 2H).
[0061] 13 C NMR (101MHz, DMSO-d 6 ( , J=22.9Hz).
[0062] 31 P NMR (162MHz, DMSO-d 6 ) δ-18.92(s).
[0063] HRMS(ESI,m / z): calcd for C 28 h 24 N 2 P[M-Cl] + 419.16716,found 419.16639.
Embodiment 3
[0065] Synthesis of bidentate phosphorus-azimidazole chloride salt ligand 5c, specifically according to the following steps:
[0066] According to the synthesis method of ligand 5a in Example 1, 82mg (1.0mmol) 1-methylimidazole was used to replace 1-metrimethylphenylimidazole, and other operating conditions were the same as in Example 1. After the reaction, 298mg of white solid was obtained. Namely, bidentate phosphorus-azaimidazole chloride salt ligand 5c, and its yield was 76%.
[0067] 1 H NMR (400MHz, DMSO-d 6 )δ8.92(s,1H),7.54-7.50(m,1H),7.47-7.43(m,2H),7.37(d,J=8.9Hz,2H),7.34-7.28(m,6H),7.02 (t, J=6.7Hz, 4H), 6.89–6.78(m, 1H), 5.57(s, 2H), 3.52(s, 3H).
[0068] 13 C NMR (101MHz, DMSO-d 6 )δ138.0, 137.8, 136.9, 136.5, 136.4, 134.8, 134.7, 134.2, 133.4, 133.2, 131.1, 131.0, 130.1, 129.7, 129.3, 128.9, 128.8, 123.6, 122.1, 51.32 (d1, J = Hz) .
[0069] 31 P NMR (162MHz, DMSO-d 6 )δ-18.90(s).
[0070] HRMS(ESI,m / z): calcd for C 23 h 22 N 2 P[M-Cl] + 357.15151,f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com