Chiral 2,4-disubstituted-thaizolidinone compounds and preparation method and application thereof in preparation of anticancer medicaments
A thiazolone and disubstituted technology, applied in the field of chemical synthesis, can solve problems such as the limitation of thiazole drugs, and achieve the effects of more research, better curative effect and easy research.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
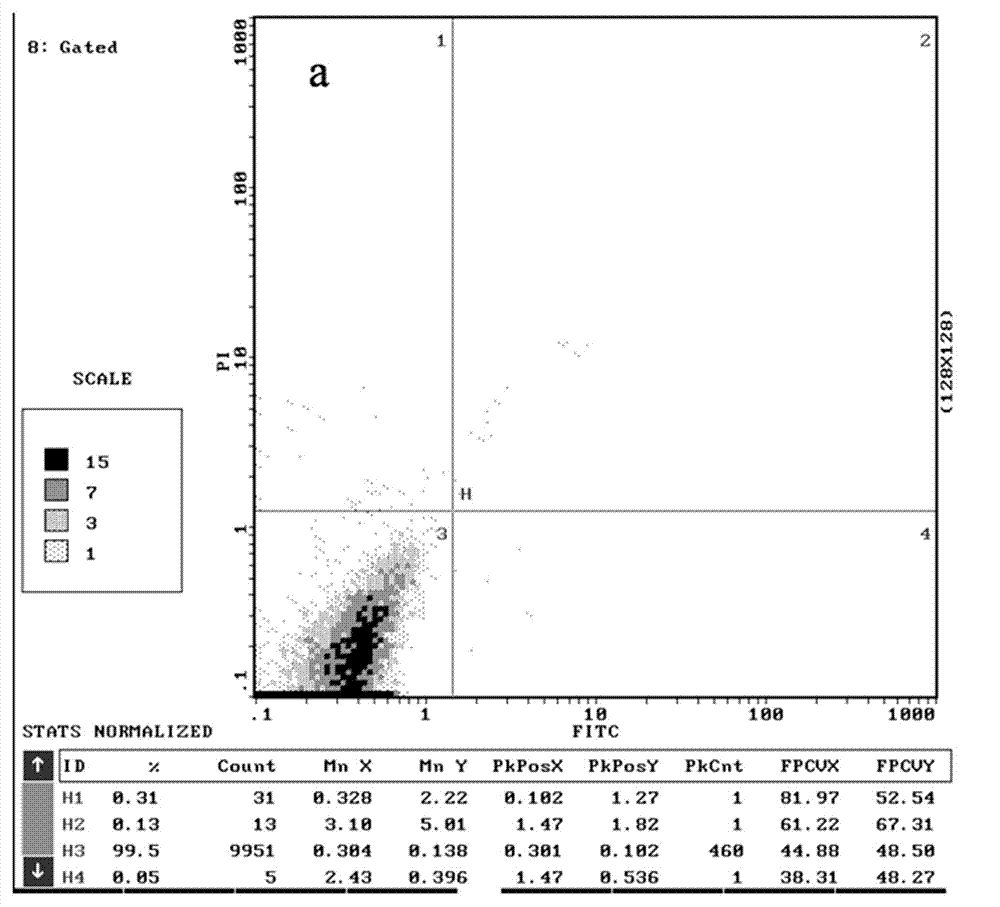
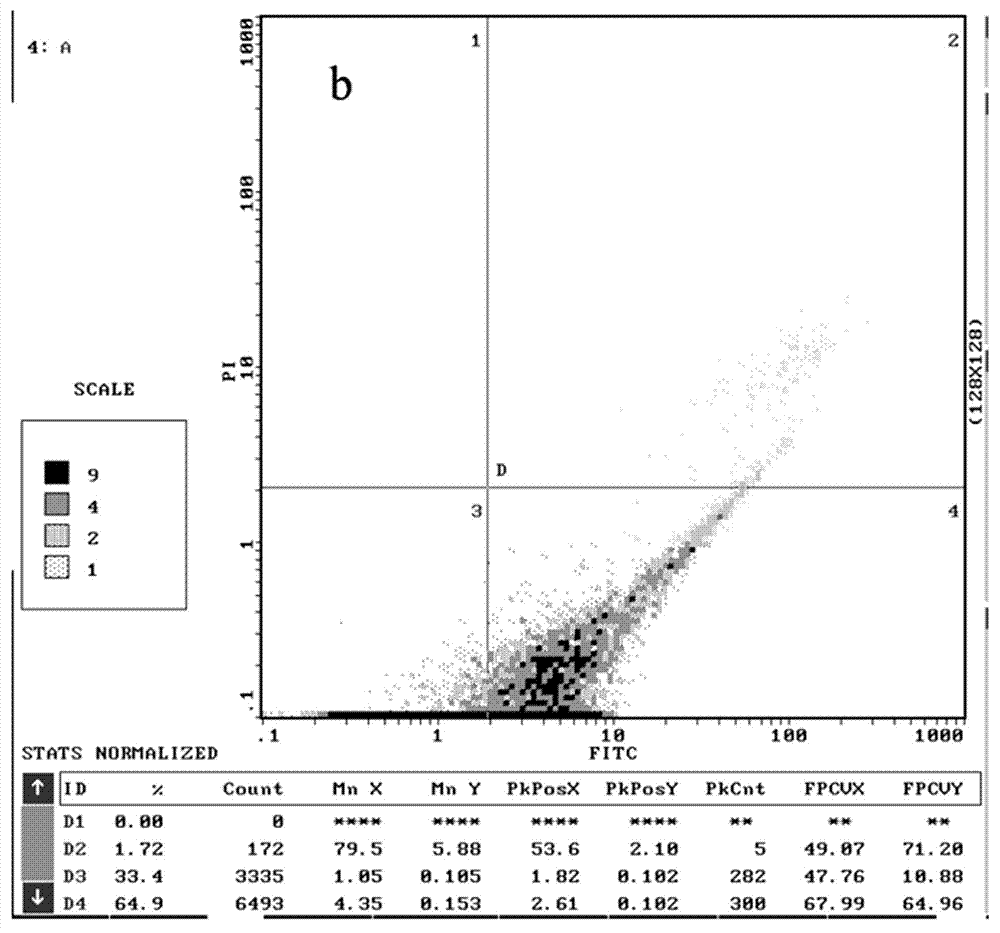
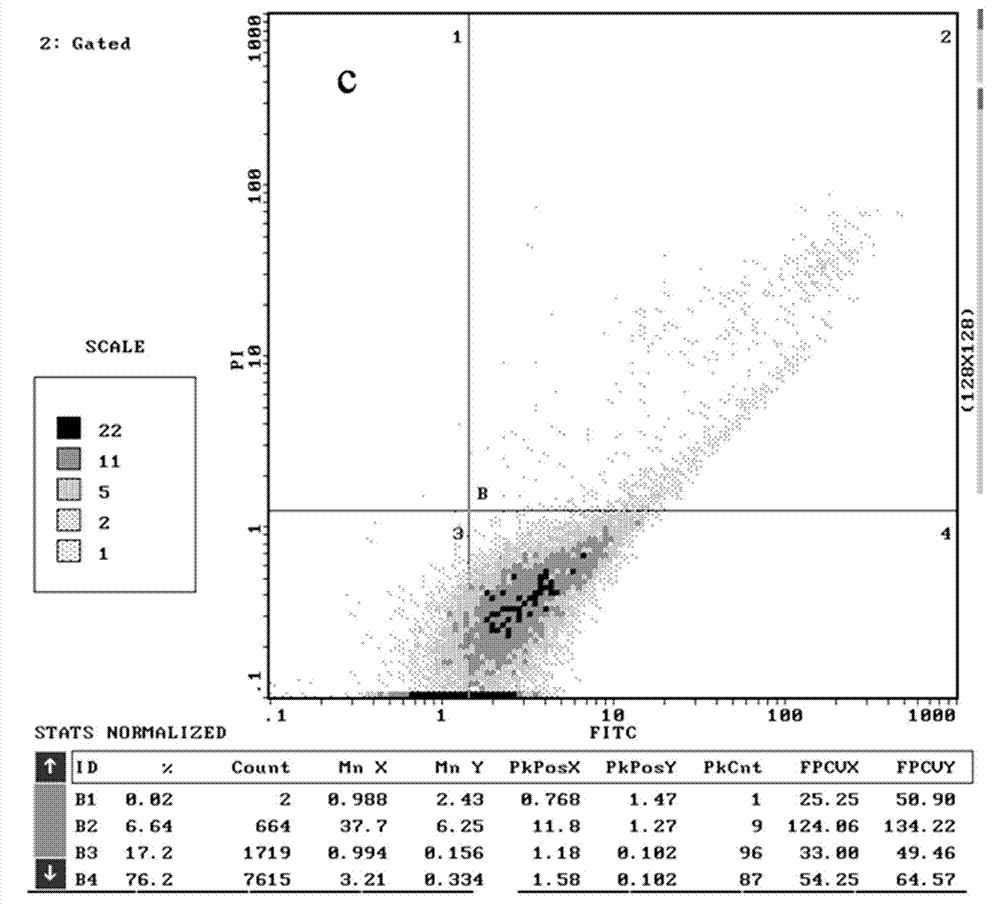
[0054] The structure and preparation of the chiral 2,4-disubstituted-thiazolone compounds of the present invention will be further illustrated by specific examples below.
[0055] In Examples 1 to 23, 2,4-disubstituted-thiazolones with different substituents were used as raw materials, and the following preparation methods were used to obtain the corresponding chiral 2,4-disubstituted-thiazolones (DT- 1~DT-23).
[0056] The preparation method of specific chiral 2,4-disubstituted-thiazolone compounds is as follows: 2,4-disubstituted-thiazolone (0.15 mmol, 1.5 eqv) and chiral ligand quinine amine-rosin thiourea (0.01 mmol, 0.1eqv) into a round-bottomed flask equipped with magnetons, add 1mL of methyl tert-butyl ether, cool in an ice bath at 0°C for 10min, then add nitrostyrene (0.1 mmol, 1eqv), at 0°C Stir the reaction, and point the high-efficiency plate to detect the completion of the reaction (6-12h). The reaction solution was concentrated and purified by silica gel column ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com