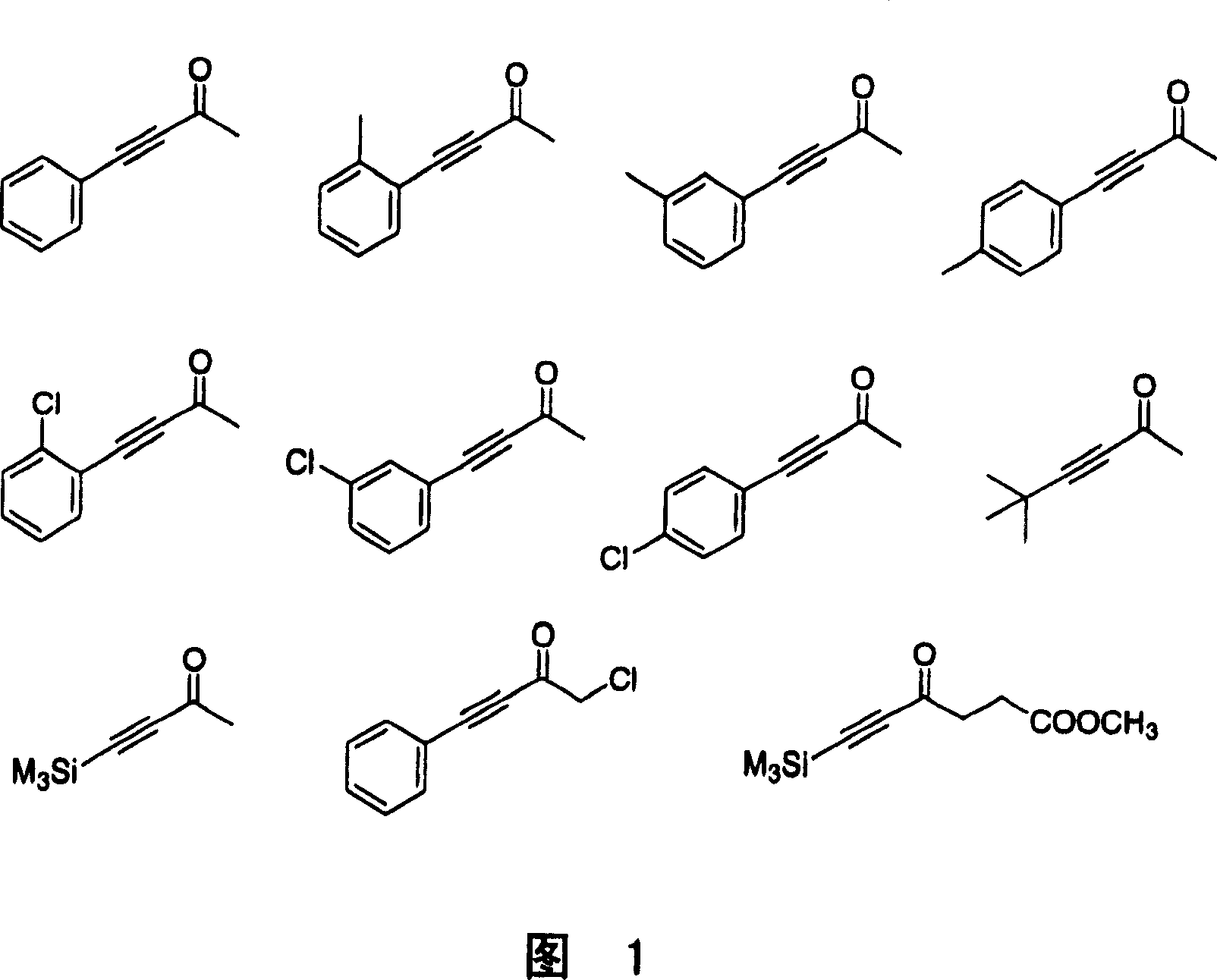
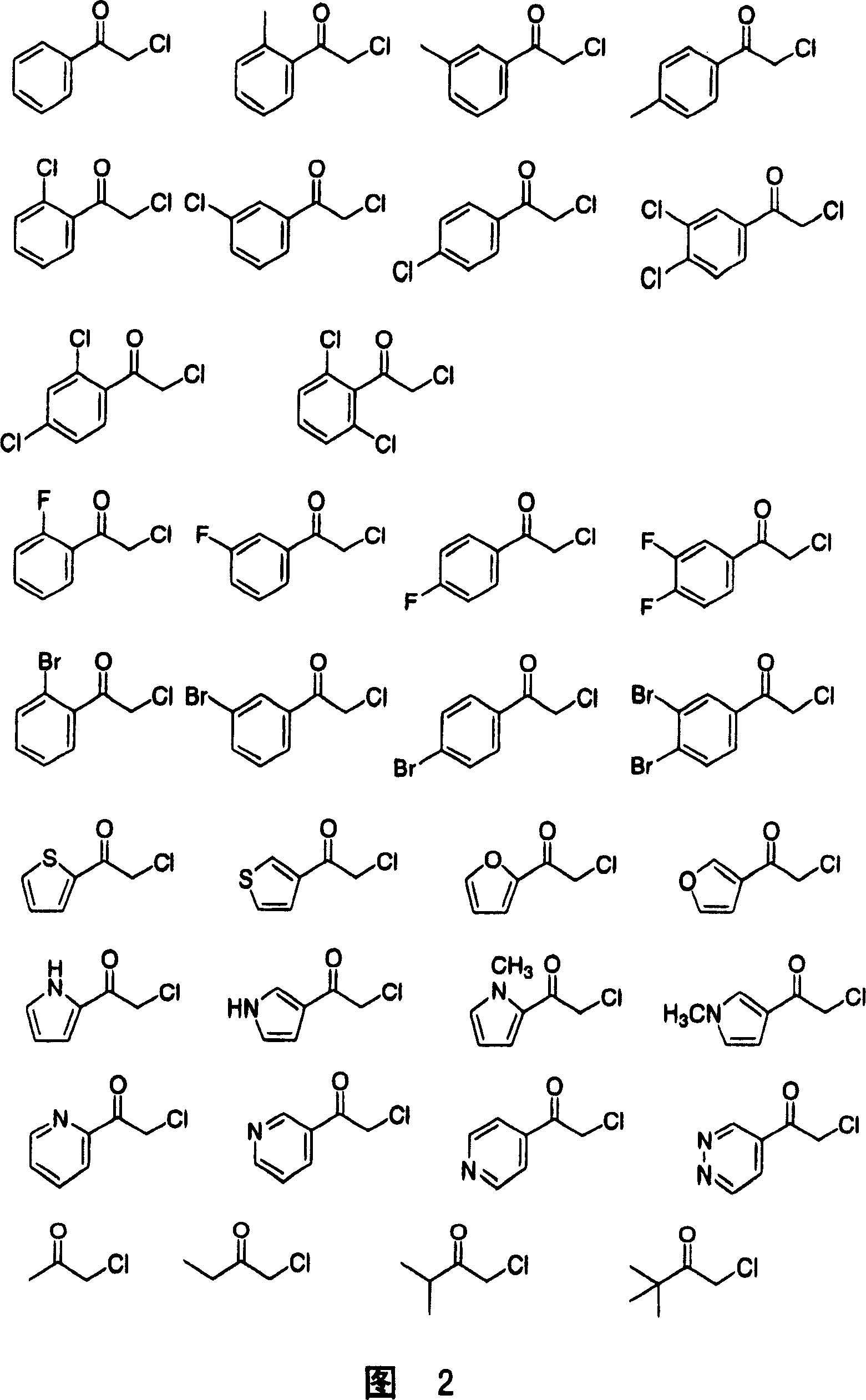
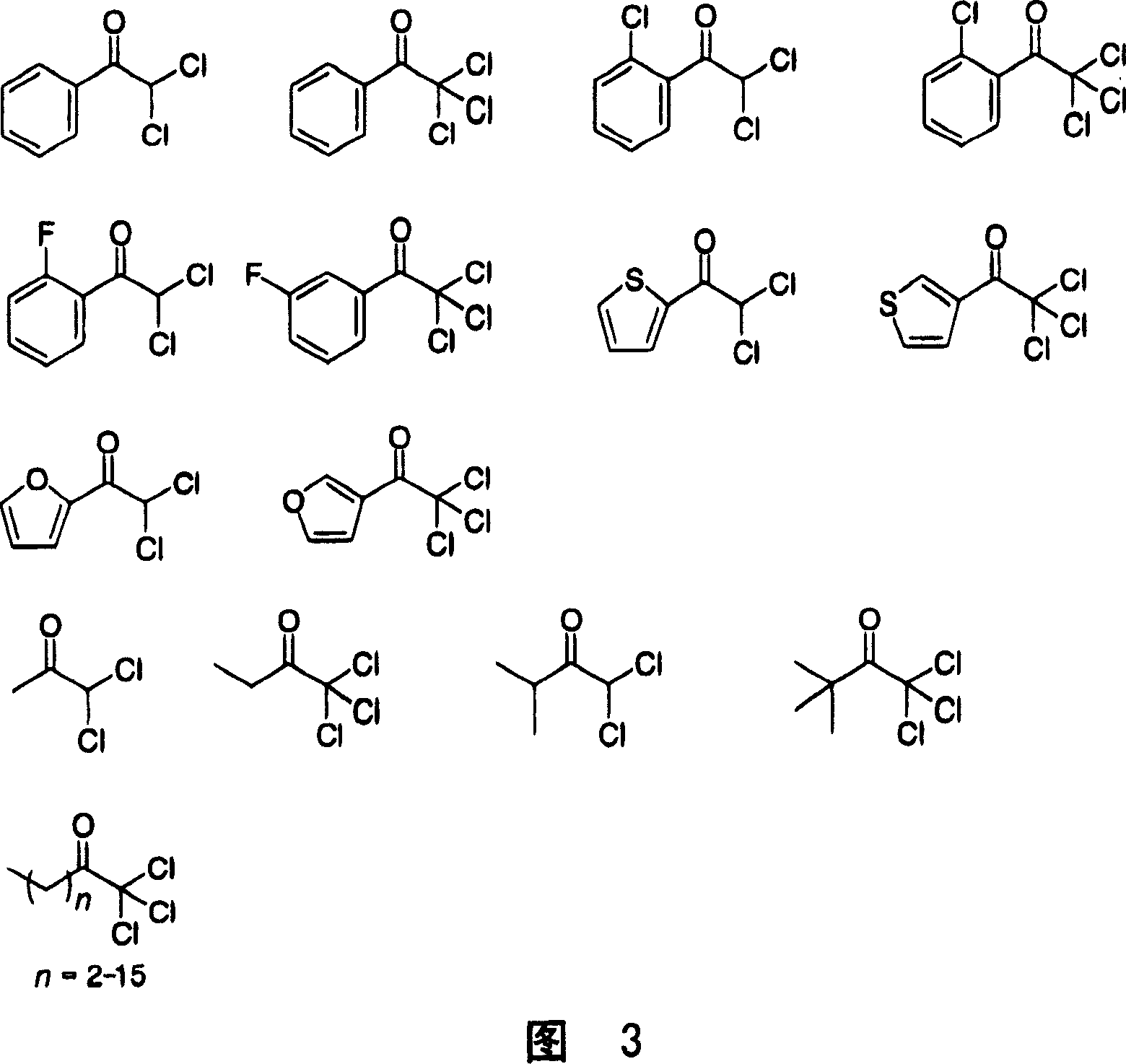
Manufacturing method of optical activity alcohol
An optically active, polar solvent technology, applied in the field of preparation of optically active alcohols, can solve the problems of low yield or enantiomeric excess, difficulty in preparing optically active alcohols, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] An example of synthesizing (S)-4-phenyl-3-butyn-2-ol by hydrogenation reaction of 4-phenyl-3-butyn-2-one will be described below. A ruthenium complex RuCl[(S,S)-Tsdpen] (p-cymene) (1.6 mg, 0.0025 mmol) was charged in a 50 mL stainless steel autoclave, and replaced with argon. Add 4-phenyl-3-butyn-2-one (0.291 mL, 2 mmol), methanol (5 mL), pressurize with hydrogen, and perform 5 displacements. Add hydrogen to 50 atmospheres to start the reaction. After stirring for 11 hours at 30°C, the reaction pressure was returned to normal pressure. From the 1 HNMR and HPLC analysis show that (S)-4-phenyl-3-butyn-2-alcohol of 90%ee is generated with a yield of 63%. The nomenclature of the ruthenium complex here starts from the left with metal atom, An anionic group, a diamine ligand, and an aromatic hydrocarbon ligand are arranged in order (refer to the following formula (4)).
[0071] Formula (4)
[0072]
Embodiment 2-10
[0076] Except for changing the catalyst and hydrogen pressure used, the reaction was carried out under the same conditions as in Example 1 to synthesize (S)-4-phenyl-3-butyn-2-ol. The results are shown in Table 1.
[0077] Table 1
[0078]
[0079] Example
[0080] Conditions: chiral ruthenium catalyst 0.0025mmol, CH 3 OH5ml, S / C=800, temperature 30°C, time 11 hours, [ketone]=0.4M, dmb: 1,4-dimethylbenzene, teb: 1,3,5-triethylbenzene, durene: 1 , 2,4,5-tetramethylbenzene, pmb: pentamethylbenzene, hmb: hexamethylbenzene.
Embodiment 11-19
[0082] Except changing the concentration of the substrate and the reaction temperature, and using additives, the reaction was carried out under the same conditions as in Example 1 to synthesize (S)-4-phenyl-3-butyn-2-ol. The results are shown in Table 2.
[0083] Table 2
[0084]
[0085] Example
[0086] Condition: CH 3 [Kone] in OH=0.4M, RuCl[(S,S)-Tsdpen](p-cymene) 0.0025mmol, S / C=800, H 2 50atm, time 11 hours, solvent 5ml, a [ketone] = 0.1M, b [ketone] = 1.0M.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com