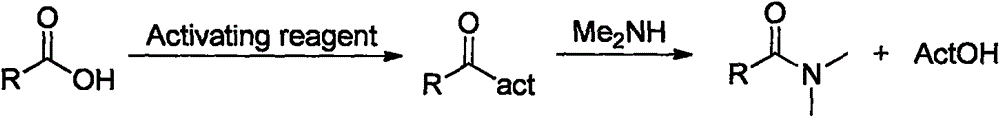
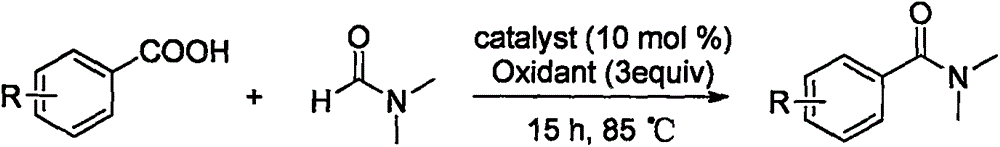
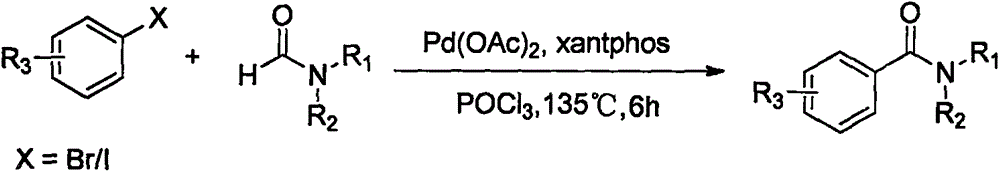Synthesis method of amide aryl compound
A kind of aryl amide, synthesis method technology, applied in the direction of preparation of organic compound, formation/introduction of amide group, chemical instrument and method etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Synthesis of benzamide (3a) from benzoic acid (1a) and DMF (2a):
[0027]
[0028] Add benzoic acid (0.036g, 0.3mmol), Xantphos (0.017g, 0.03mmol), K 2 S 2 o 8 (0.162g, 0.6mmol), Ru(p-cymene)Cl 2 (0.0092g, 0.015mmol), DMF (2mL), fill it with argon gas, tighten the bottle cap, and seal the reaction at an external temperature of 160°C for 12h; monitor the complete reaction by gas chromatography; filter the reaction solution, remove the solvent by rotary evaporation, and pass through the column After separation by chromatography (ethyl acetate:n-hexane=1:1), a colorless transparent liquid 3a was obtained with a yield of 88%.
[0029] 1 H NMR (300MHz, CDCl 3 )δ7.39(s, 5H), 3.10(s, 3H), 2.96(s, 3H); 13 C NMR (75MHz, CDCl 3 )δ171.76(s), 136.34(s), 129.61(s), 128.43(s), 127.11(s), 39.69(s), 35.43(s); MS(70eV, EI) m / z(EI) C 9 h 11 NO[M]: 149.19, 51(36), 77(100), 105(29), 148(56), 149(5).
Embodiment 2
[0031] Synthesis of 3-methylbenzamide (3b) from 3-methylbenzoic acid (1b) and DMF (2a):
[0032]
[0033] Add 3-methylbenzoic acid (0.041g, 0.3mmol), Xantphos (0.017g, 0.03mmol), K 2 S 2 o 8 (0.162g, 0.6mmol), Ru(p-cymene)Cl 2 (0.0092g, 0.015mmol), DMF (2mL), fill it with argon gas, tighten the bottle cap, and seal the reaction at an external temperature of 160°C for 12h; monitor the complete reaction by gas chromatography; filter the reaction solution, remove the solvent by rotary evaporation, and pass through the column After separation by chromatography (ethyl acetate:n-hexane=1:1), a colorless transparent liquid 3b was obtained with a yield of 90%.
[0034] 1 H NMR (300MHz, CDCl 3 )δ7.23(dq, J=13.0, 7.4Hz, 4H), 3.04(d, J=39.5Hz, 6H), 2.37(s, 3H); 13 C NMR (75MHz, CDCl3 )δ171.94(s), 138.28(s), 136.37(s), 130.28(s), 128.24(s), 127.73(s), 124.05(s), 39.68(s), 35.38(s), 21.45( s); MS (70eV, EI) m / z (EI) C 10 h 13 NO[M]: 163.22, 65(27), 91(100), 119(33), 162(28), 16...
Embodiment 3
[0036] Synthesis of 4-hydroxybenzamide (3c) from 4-hydroxybenzoic acid (1c) and DMF (2a):
[0037]
[0038] Add 4-hydroxybenzoic acid (0.041g, 0.3mmol), Xantphos (0.017g, 0.03mmol), K 2 S 2 o 8 (0.162g, 0.6mmol), Ru(p-cymene)Cl 2 (0.0092g, 0.015mmol), DMF (2mL), fill it with argon gas, tighten the bottle cap, and seal the reaction at an external temperature of 160°C for 12h; gas chromatography monitors that the reaction is complete; filter the reaction solution, remove the solvent by rotary evaporation, and pass through the column After separation by chromatography (ethyl acetate:n-hexane=1:1), a white solid 3c was obtained with a yield of 83%.
[0039] 1 H NMR (300MHz, CDCl 3 )δ7.25-7.19 (m, 2H), 6.74-6.66 (m, 2H), 3.06 (d, J=19.0Hz, 6H); 13 C NMR (75MHz, CDCl 3 )δ172.82(s), 158.77(s), 129.20(s), 126.31(s), 115.50(s), 38.04(d, J=321.6Hz); MS(70eV, EI) m / z(EI) C 9 h 11 NO 2 [M]: 165.19, 63(26), 65(100), 73(44), 93(83), 121(82), 164(28), 165(3).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com