Ruthenium complex containing ortho-carbonboryl benzoxazole structure and preparation and application thereof
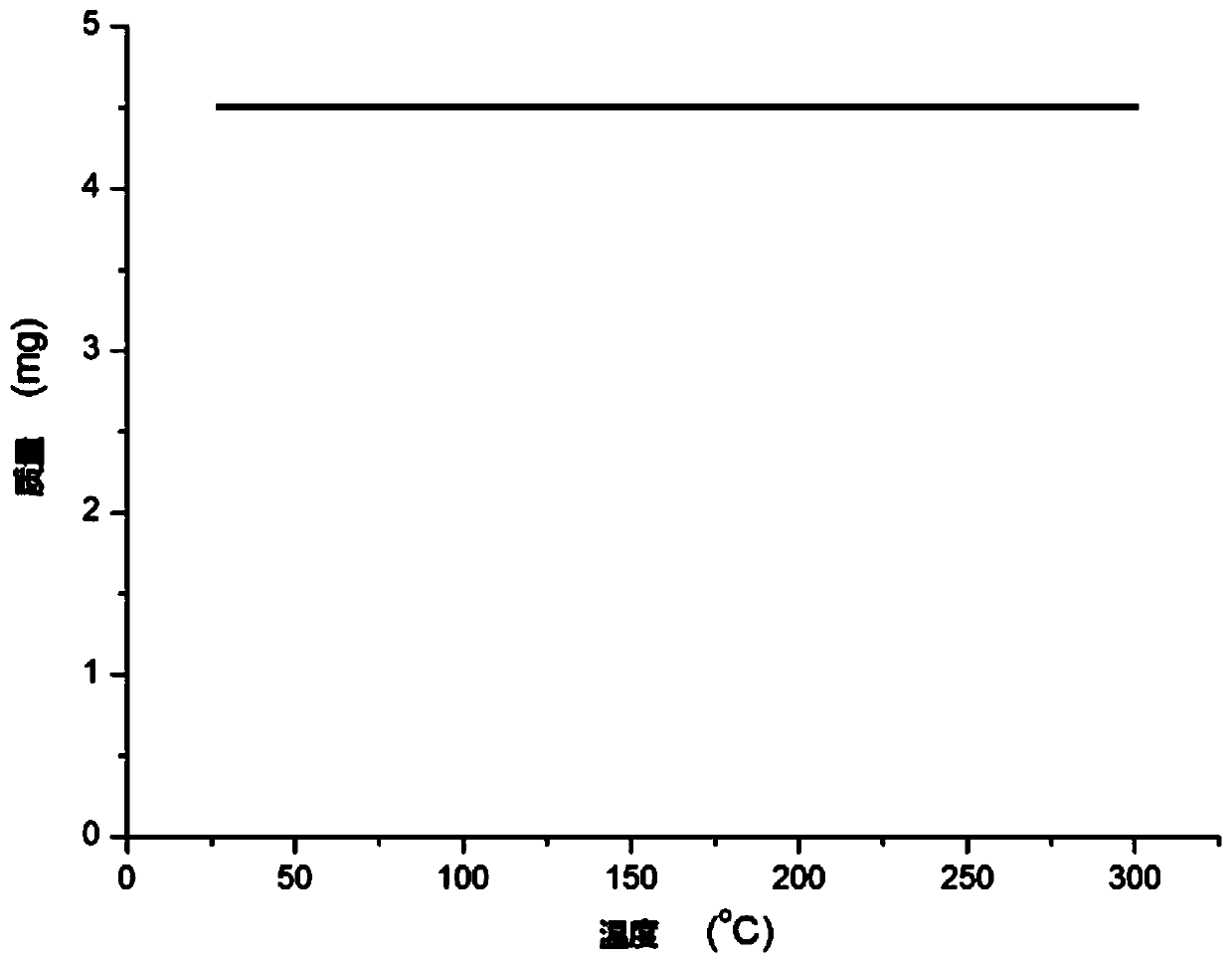
A technology of carboryl benzoxazole and ruthenium complexes, which is applied in the field of semi-sandwich ruthenium complexes and their preparation, can solve problems such as environmental impact, expensive iridium complexes, and highly toxic selenium compounds, and achieve stable thermal Stability, the preparation method is simple and green, and the effect of stabilizing physical and chemical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
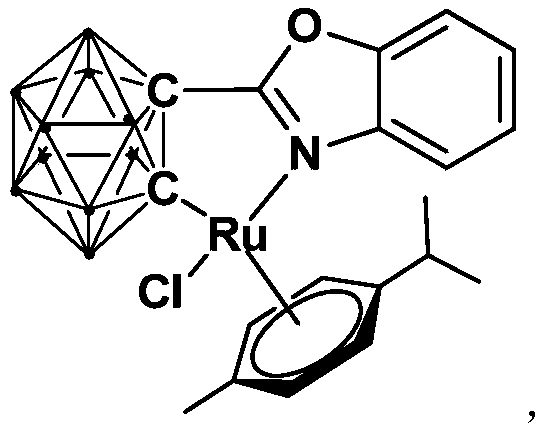
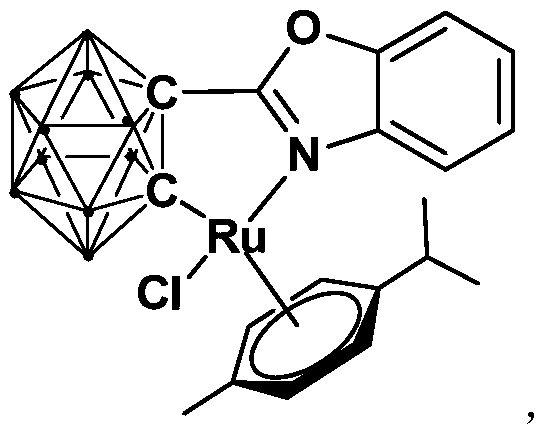
[0031] Synthesis of semi-sandwich ruthenium complexes Ru with ortho carboryl benzoxazole structure:
[0032]
[0033] Among them, "·" represents the boron-hydrogen bond B-H.
[0034] At -78°C, n-BuLi (1.6M) in n-hexane (1.00 mL, 1.6 mmol) was slowly added dropwise to the o-C 2 B 10 h 10 (92.0mg, 0.64mmol) in tetrahydrofuran solution, stirred at this temperature for 30 minutes, slowly rose to room temperature and continued to react for 1 hour, then added bromobenzoxazole (126.7mg, 0.64mmol), continued to react at room temperature 6 hours. Then the binuclear ruthenium compound [(p-cymene)RuCl 2 ] 2 (256.0 mg, 0.32 mmol) was added to the reaction system for an additional 3 hours. After the reaction, stand and filter, and dry the solvent under reduced pressure. The obtained crude product is separated by column chromatography (petroleum ether / tetrahydrofuran=6:1) to obtain the orange-red target product ruthenium (II) complex Ru (241.2 mg, Yield 71%).
[0035] 1 H NMR (4...
Embodiment 2
[0038] Ruthenium(II) complexes catalyze the autoxidative coupling reaction of primary amines:
[0039]
[0040] The ruthenium complex prepared in Example 1 was used as a catalyst to catalyze the autoxidative coupling reaction of primary amines: to benzylamine (10mmol, 1.07g) was added an ortho carborane o-C 2 B 10 h 10 Divalent ruthenium complex (0.002mmol, 2.6mg) in toluene solution, and air was introduced as an oxidant to react, the reaction temperature was 30°C, and the reaction time was 180 minutes. After the end, the concentrated reaction solution was directly separated by silica gel column chromatography , dried until the mass remains unchanged, and the corresponding imine compound C 14 h 13 N (yield 86%), 1 H NMR (400MHz, CDCl 3):δ=8.32(s,1H),7.72-7.69(m,2H),7.35-7.33(d,J=1.8Hz,3H),7.27-7.26(d,J=4.4Hz,4H),7.20- 7.17 (m, 1H), 4.75 (s, 2H), elemental analysis: C86.12, H 6.71, N 7.17 (theoretical); C 86.03, H 6.69, N 7.12 (actual).
Embodiment 3
[0042] Ruthenium(II) complexes catalyze the autoxidative coupling reaction of primary amines:
[0043]
[0044] The ruthenium complex prepared in Example 1 was used as a catalyst to catalyze the autoxidative coupling reaction of primary amines: in 4-methylbenzylamine (10mmol, 1.37g), add an ortho carborane o-C 2 B 10 h 10 Divalent ruthenium complex (0.002mmol, 2.6mg) in toluene solution, and air was introduced as an oxidant to react, the reaction temperature was 30°C, and the reaction time was 60 minutes. After the end, the concentrated reaction solution was directly separated by silica gel column chromatography , dried until the mass remains unchanged, and the corresponding imine compound C 16 h 17 N (93% yield), 1 H NMR (400MHz, CDCl 3 ):δ=8.38(s,1H),7.70-7.64(d,J=6.8Hz,2H),7.39-7.36(d,J=4.0Hz,2H),7.30-7.18(m,4H),4.75( s, 2H), 2.41 (s, 3H), 2.35 (s, 3H), elemental analysis: C86.05, H 7.67, N 6.27 (theoretical); C 86.10, H 7.69, N 6.30 (actual).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com