Preparation method and application of non-natural ginsenoside
A ginsenoside, non-natural technology, applied in the fields of biotechnology and botany, can solve problems such as high price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
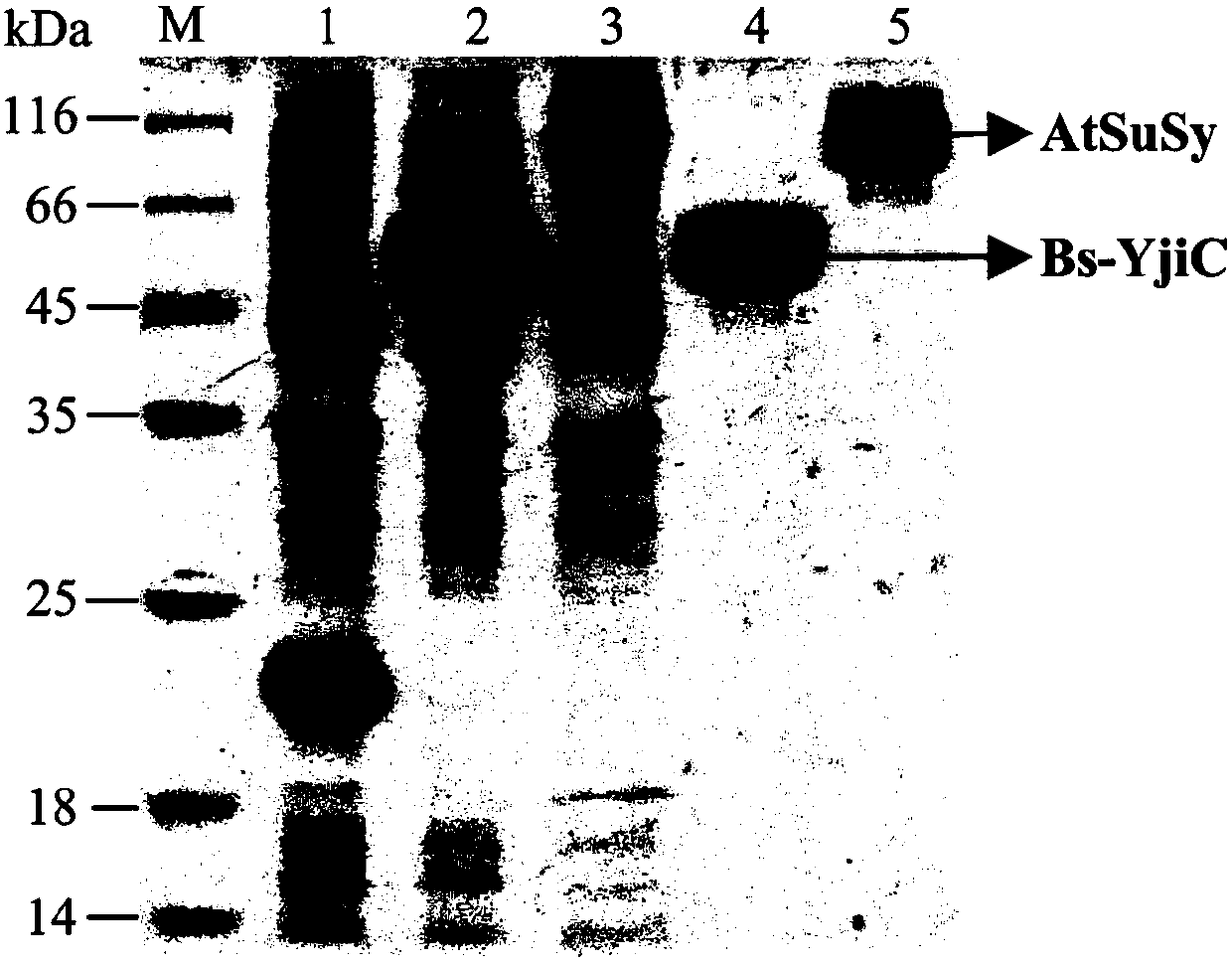
[0069] Cloning, expression and purification of embodiment 1 glycosyltransferase and sucrose synthase
[0070] The glycosyltransferase in the patent of the present invention is screened from Bacillus subtilis 168, the amino acid sequence is shown in SEQ ID NO.1, and the nucleotide sequence is shown in SEQ ID NO.7.
[0071] The nucleic acid sequence of said SEQ ID NO.7 is as follows:
[0072] ATGAAAAAGTACCATATTTCGATGATCAATATCCCGGCGTACGGACATGTCAATCCTACGCTTGCTTTAGTAGAGAAGCTTTGTGAGAAAGGGCACCGTGTCACGTACGCGACGACTGAGGAGTTTGCGCCCGCTGTTCAGCAAGCCGGTGGAGAAGCATTGATCTATCATACATCCTTGAATATTGATCCTAAGCAAATCAGGGAGATGATGGAAAAGAATGACGCGCCCCTCAGCCTTTTGAAAGAATCACTCAGCATTCTGCCGCAGCTTGAGGAGTTATATAAGGATGATCAGCCTGATCTGATCATCTATGACTTTGTTGCGCTGGCTGGTAAATTGTTTGCTGAAAAGCTTAATGTTCCGGTCATTAAGCTCTGTTCGTCATATGCCCAAAATG AATCCTTTCAGTTAGGAAATGAAGACATGCTGAAAAAAATAAGAGAAGCAGAGGCTGAATTTAAAGCCTACTTGGAGCAAGAGAAGTTGCCGGCTGTTTCATTTGAACAGTTAGCTGTGCCGGAAGCATTAAATATTGTCTTTATGCCGAAGTCTTTTCAGATTCAGCATGAGACGTTCGATGACCGTTTCTGTTTT...
Embodiment 2
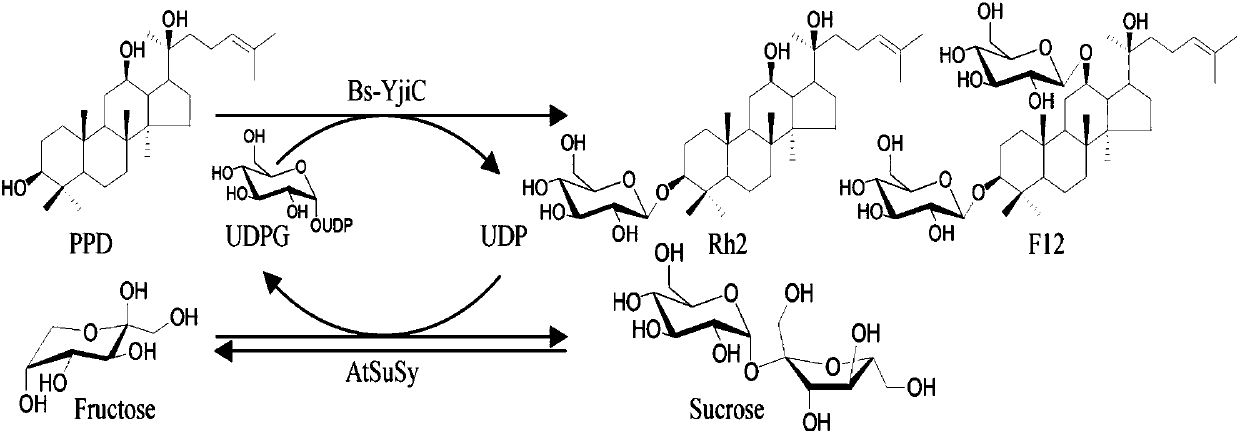
[0085] Example 2 Coupling of glycosyltransferase Bs-YjiC and sucrose synthase AtSuSy to catalyze the synthesis of ginsenosides Rh2 and F12 from protopanaxadiol
[0086] Using protopanaxadiol (PPD) as the substrate and cheap sucrose as the glycosyl donor, it can catalyze the C3-OH and C12-OH glycosylation of PPD through the coupling reaction of glycosyltransferase-sucrose synthase Ginsenoside Rh2 (3-O-β-D-glucopyranosyl-20(S)-protopanaxadiol) and unnatural ginsenoside F12 (3-O-β-D-glucopyranosy-12-β-D-glucopyranosyl-protopanaxadiol), specifically react as figure 2 As shown, the details are as follows:
[0087]
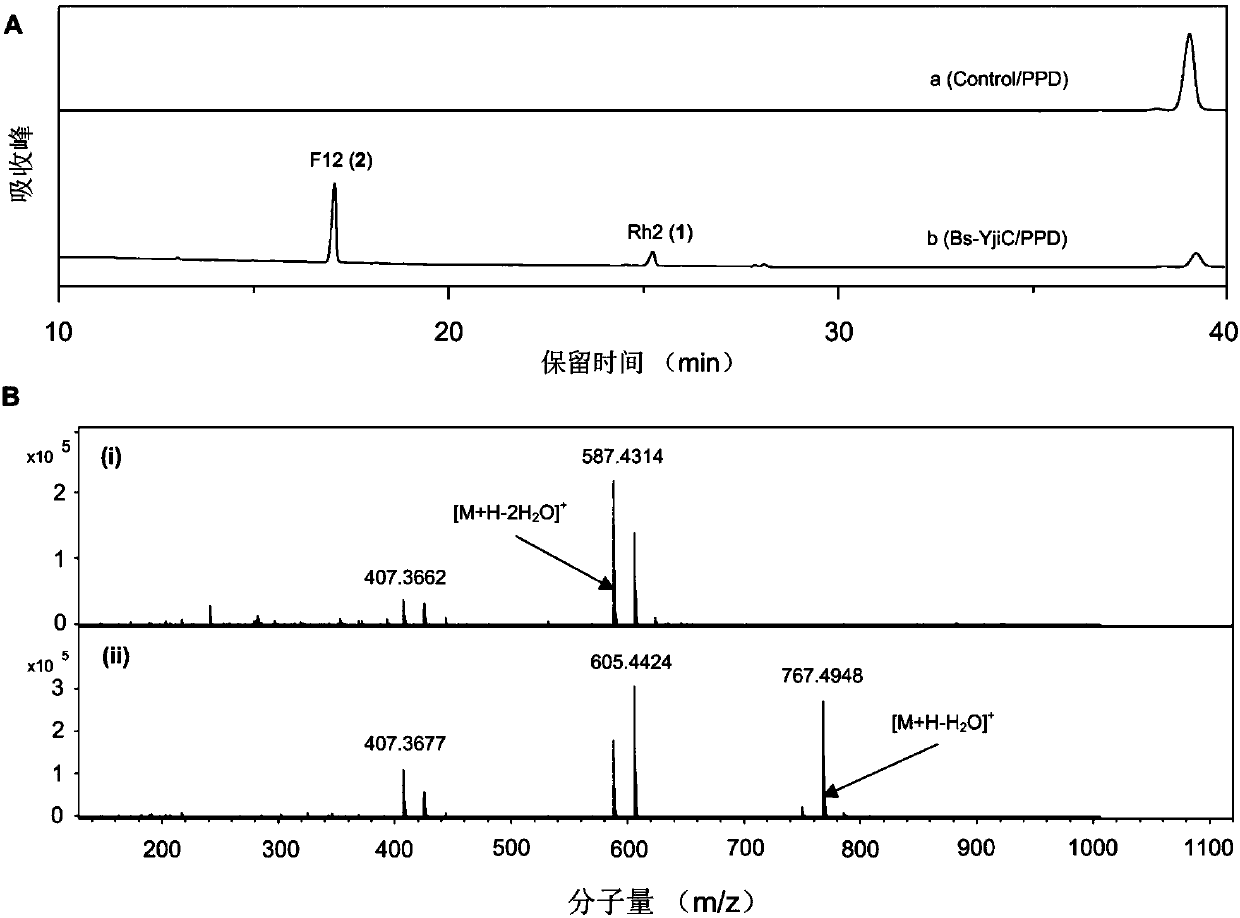
[0088] The enzyme reaction system includes: 2mM protopanaxadiol (PPD), 0.5mM UDP, 300mM sucrose, 160mU / mL glycosyltransferase Bs-YjiC and 200mU / mL sucrose synthase AtSuSy, react at 35°C for 0.5h. The enzyme reaction product was identified by high performance liquid chromatography-electrospray ionization mass spectrometry (HPLC-ESI-MS), and the results were as foll...
Embodiment 3
[0091] Example 3 Feed-batch Synthesis of Ginsenoside Rh2 and F12
[0092] In view of the fact that the coupling reaction of glycosyltransferase Bs-YjiC and sucrose synthase AtSuSy can glycosylate 2mM protopanaxadiol into ginsenoside Rh2 and non-natural ginsenoside F12 within 0.5h, so further batch feeding, That is, protopanaxadiol was supplemented with 2mM every 2h to further explore the potential of the glycosylation method to catalyze the synthesis of ginsenosides Rh2 and F12 from protopanaxadiol. The results are as follows: image 3 shown.
[0093] From Figure 4 It can be seen that through 4 batches of supplementing protopanaxadiol substrates, about 8 hours, 1.6mM ginsenoside Rh2 (1.0g / L) and 8.3mM ginsenoside F12 (6.5g / L) can be obtained finally. The synthesis rates of Rh2 and F12 exceeded 0.125g / L / h and 0.8g / L / h. At present, Saccharomyces cerevisiae engineering bacteria can only produce 15-300mg / L ginsenosides after 7 days of fermentation, which is far lower than the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com