Dendrimer with alkynyl core and outer amino acid shell, and Huisgen 1,3-dipolar cycloaddition synthetic method and application thereof
A technology of dendrimers and amino acids, applied in chemical instruments and methods, other chemical processes, organic chemistry, etc., can solve problems such as structural defects of dendrimers, difficulty in dendrimer purification, easy side reactions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
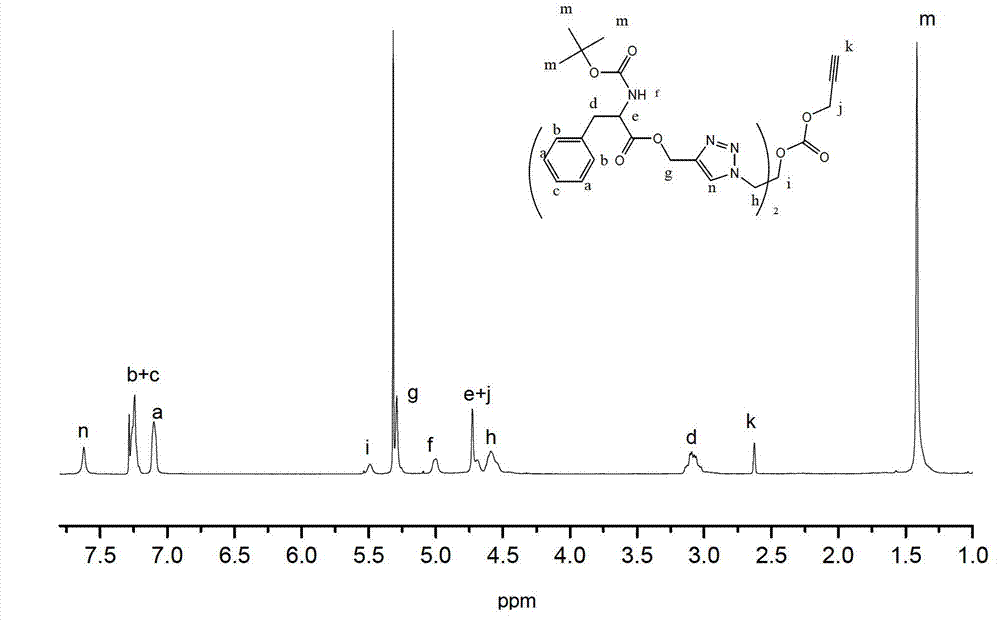
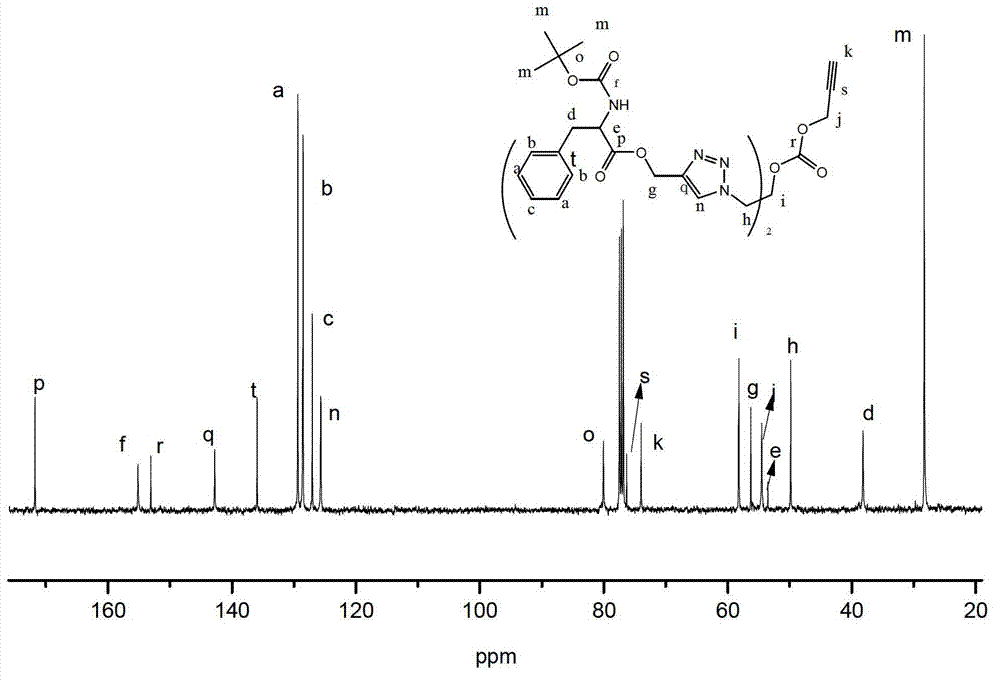
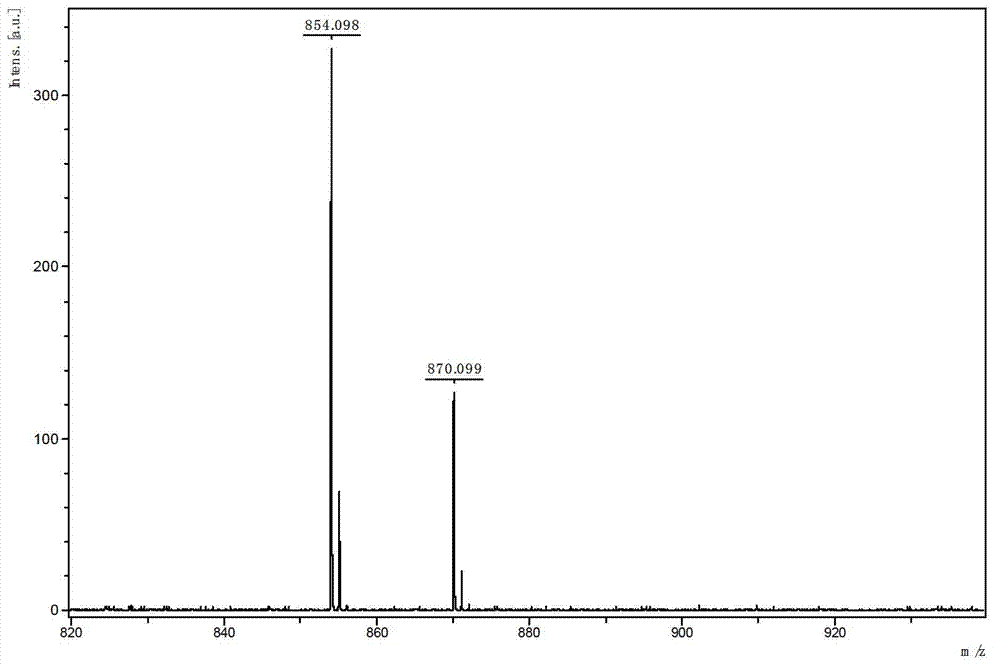
[0093] Embodiment 1: Synthetic method of dendrimers whose inner core is alkynyl and outer layer is phenylalanine
[0094] (1) Synthesis of dendrimers whose outer layer is azide group
[0095] (a) Synthesis of 1,3-diazido-2-propanol (C-1)
[0096] Dissolve 2.356g (36.2mmol) sodium azide in 15.0ml H 2 O, 1.340g (14.5mmol) epichlorohydrin dissolved in 15.0ml acetonitrile; mix the two, react at 85±3°C for 36 hours, remove the acetonitrile in the reaction system by rotary evaporation; extract once with 50ml ethyl acetate , and then extracted three times with 25ml of water; the extracted oil phase was extracted with anhydrous MgSO 4 After drying, suction filtration and distillation yielded 1.466 g of compound 1,3-diazido-2-propanol (C-1). (b) Generation 0.5 dendrimers with an azide-based outer layer (G0.5-N 3 )Synthesis
[0097] Dissolve 1.552g (7.7mmol) p-nitrophenyl chloroformate and 0.998g (7.0mmol) 1,3-diazido-2-propanol in 5.0ml dichloromethane and 7.0ml dichloromethane, r...
Embodiment 2
[0134] Example 2: Synthesis of a dendrimer whose inner core is an alkyne group and whose outer layer is L-histidine
[0135] (1) Synthesis of dendrimers whose outer layer is azide group
[0136] (a) Synthesis of 1,3-diazido-2-propanol (C-1)
[0137] Dissolve 1.177g (18.1mmol) sodium azide in 15.0ml H 2 In O, 0.837g (9.0mmol) of epichlorohydrin was dissolved in 15.0ml of acetonitrile; the two were mixed and reacted at 95°C for 48 hours; the acetonitrile in the reaction system was removed by rotary evaporation; then, extracted with 60ml of ethyl acetate once, then extracted three times with 25ml water; the oil phase after extraction was extracted with anhydrous MgSO 4 After drying, suction filtration and rotary evaporation, 0.611 g of 1,3-diazido-2-propanol was obtained.
[0138] (b) Generation 0.5 dendrimers with an azide-based outer layer (G0.5-N 3 )Synthesis
[0139] Dissolve 2.821g (14.0mmol) p-nitrophenyl chloroformate and 0.994g (7.0mmol) 1,3-diazido-2-propanol in 5.0...
Embodiment 3
[0159] Example 3: Synthesis of dendrimers whose inner core is alkynyl and outer layer is tryptophan
[0160] (1) Synthesis of dendrimers whose outer layer is azide group
[0161] (a) Synthesis of 1,3-diazido-2-propanol (C-1)
[0162] Dissolve 3.059g (47.1mmol) sodium azide in 15.0ml H 2 In O, 1.455g (15.7mmol) of epichlorohydrin was dissolved in 15.0ml of acetonitrile; the two were mixed and reacted at 95°C for 24 hours; the acetonitrile in the reaction solution was removed by rotary evaporation; then, 60ml of ethyl acetate was used to Extract once, and then extract three times with 25ml of water. The extracted oil phase was anhydrous MgSO 4 After drying, suction filtration and vacuum distillation, 1.576 g of compound 1,3-diazido-2-propanol was obtained.
[0163] (b) Generation 0.5 dendrimers with an azide-based outer layer (G0.5-N 3 )Synthesis
[0164] Dissolve 1.919g (9.5mmol) of p-nitrophenyl chloroformate and 1.352g (9.5mmol) of 1,3-diazido-2-propanol (C-1) in 5.0ml ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com