Method for preparing hesperetin from enzymatic hydrolysis neohesperidin or hesperidin
A new hesperidin, enzymatic hydrolysis technology, applied in the field of bioengineering, can solve the problems of complex reaction product types and components, affecting yield, difficult purification and separation, etc., to achieve high product purity, high conversion efficiency, and high application value. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Preparation of crude enzyme solution
[0022] Use 3% wheat bran extract, 0.2% yeast extract, 1% glucose, 0.5% KH2PO4, 0.2% (NH4)2SO4 as the seed medium, and distribute 50ml of the above seed medium into 100ml Erlenmeyer flasks , sterilized at 120° C. for 15 minutes, inoculated the agar plate culture of Penicillium decumbens into it, and cultured at 30° C. for 2 days as a seed solution.
[0023] 500ml Erlenmeyer culture bottle is put into 100ml containing 5% corn steep liquor, 0.2% yeast extract, 0.2% KH2PO4, the culture medium of 1% naringin, pH4.5, autoclave 121 degree 30min. Inoculate 1ml of the seed liquid into this culture medium, culture at 150rpm, shaker at 25°C for 5 days. Centrifuge to collect the supernatant, which is the crude enzyme solution.
Embodiment 2
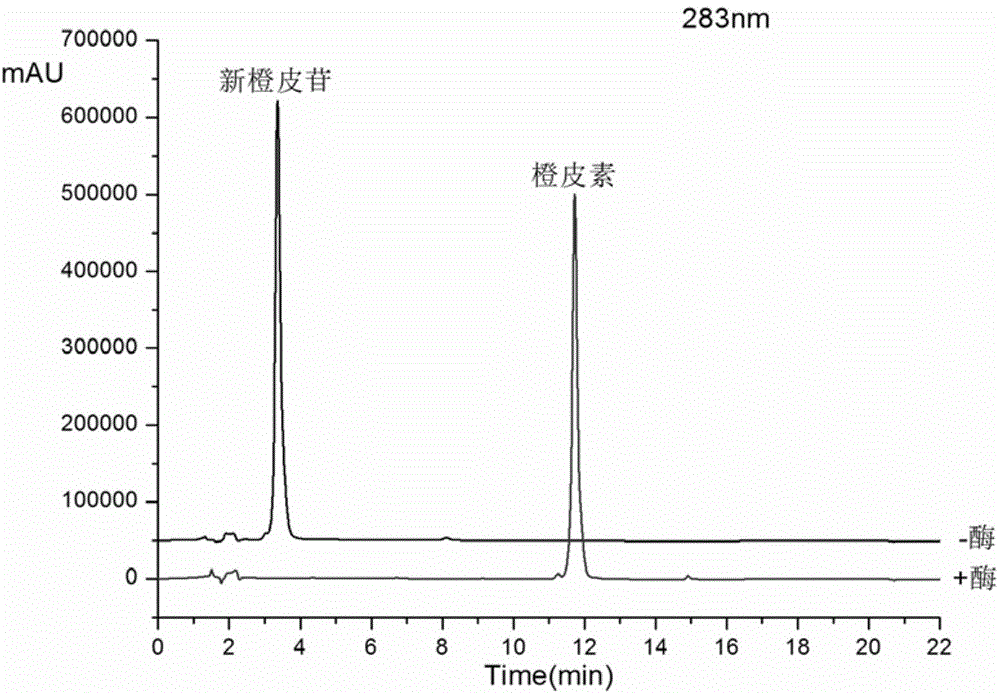
[0025] Take a 100ml Erlenmeyer flask and add 20ml of phosphate buffer solution (pH6.0), 3.5ml of crude enzyme solution, 250ul of 20mM activator, 600ul of 80mM neohesperidin solution, seal the bottle mouth, and store at 37°C 200rpm rotary shaker reaction 4h, every 1h sampling for HPLC analysis (detection wavelength 283nm, mobile phase: acetonitrile (B) / pure water (A), gradient is, 0-10min, 25%-50%B; 10.0 -12.0min, 50%-90%B; 12.0-16.0min, 90%-90%B, flow rate 1ml / min), the conversion rate of neohesperidin was greater than 98% in 0.5h, and completely converted after 4h of reaction. The prepared product is hesperetin. Such as figure 1 shown by figure 1 It can be seen that the crude enzyme solution has a very high conversion rate to neohesperidin.
Embodiment 3
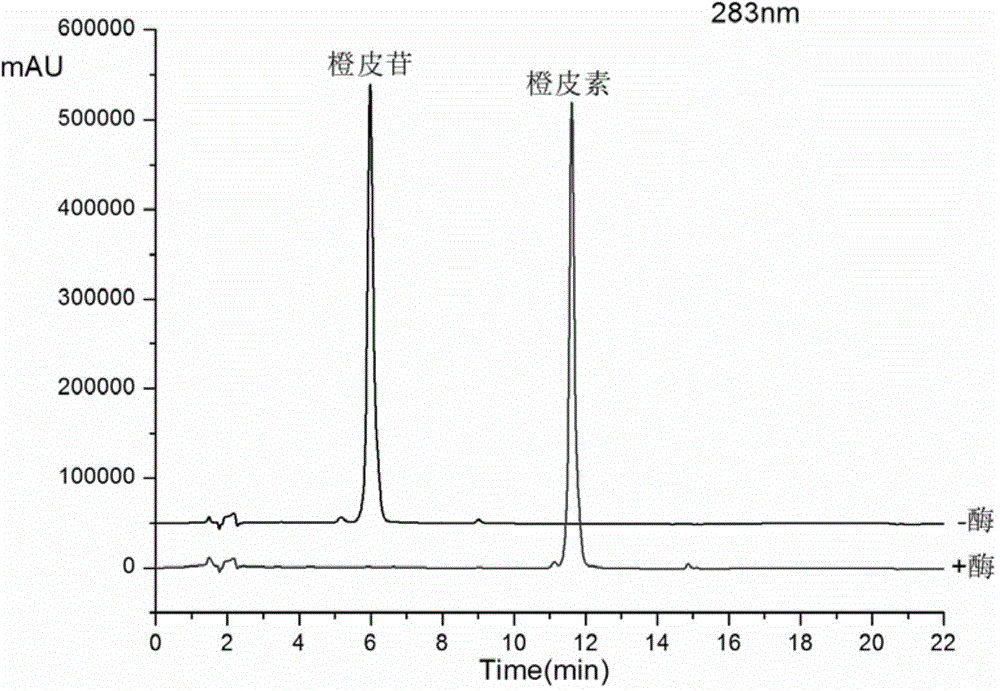
[0027] Get 100ml Erlenmeyer flask and add 20ml phosphate buffer (pH6.0) successively, 3.5ml concentration is the enzyme solution of 100mg / ml, 250ul concentration is the activator of 20mM, 600ul concentration is the hesperidin solution of 80mM, after closing the bottle mouth, Reaction on a rotary shaker at 200 rpm at 37°C for 4 hours, sampling every 1 hour for HPLC analysis (detection wavelength 283nm, mobile phase: acetonitrile (B) / pure water (A), gradient 0-10min, 25%-50 %B; 10.0-12.0min, 50%-90%B; 12.0-16.0min, 90%-90%B, flow rate 1ml / min), the conversion rate of hesperidin was greater than 98% in 0.5h, and completely converted after 4h of reaction. The prepared product is hesperetin. Such as figure 2 shown by figure 2 It can be seen that the crude enzyme solution has a significant advantage in converting hesperidin to prepare hesperetin.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com