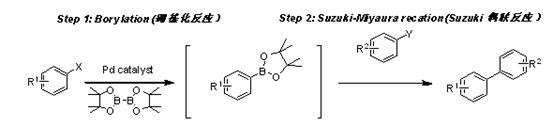
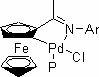Application of cyclopalladated ferrocenylimine-phosphine adduct in synthesis of asymmetric biaryl compound
A ferrocene imine ring palladium, adduct technology, applied in the preparation of organic compounds, the preparation of amino compounds from amines, organic compounds/hydrides/coordination complex catalysts, etc. To obtain the ideal yield, difficult separation of the target product, etc., to achieve the effects of mild reaction conditions, wide applicability, and strong reaction specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Preparation of 4-acetylbiphenyl
[0030] Take 0.012 mmol of ferroceneimine cyclopalladium-tricyclohexylphosphine adduct, 0.72 mmol of pinacol diboronate, and 1.2 mmol of KOAc into a 10 ml Schlenk tube, and repeatedly vacuumize and fill with N 2 5 times. N 2 Add 0.6 mmol bromobenzene and 2 ml 1,4-dioxane under protection. Stir at room temperature for 5 min, then place in an oil bath heated to 100 °C, and react for 2 h. Suspend the reaction and cool to room temperature. N 2 Add 4-bromoacetophenone 0.5 mmol, 1 ml 1,4-dioxane, 0.5 ml K 3 PO 4 aqueous solution (5M), heated to 100°C, and continued to react for 3 h. Stop the reaction and cool to room temperature. The reaction solution was diluted with ethyl acetate, filtered, washed once with water, and the organic phase was washed with Na 2 SO 4 Dry, filter and concentrate. The residue was separated by thin-layer chromatography using ethyl acetate / petroleum ether=1 / 20 as a developing solvent to obtain 91 mg of the ...
Embodiment 2
[0032] Preparation of 4-acetylbiphenyl
[0033] Take 0.012 mmol of ferroceneimine cyclopalladium-tricyclohexylphosphine adduct, 0.72 mmol of pinacol diboronate, and 1.2 mmol of KOAc into a 10 ml Schlenk tube, and repeatedly vacuumize and fill with N 2 5 times. N 2 Add 0.6 mmol bromobenzene and 2 ml 1,4-dioxane under protection. Stir at room temperature for 5 min, then place in an oil bath heated to 100 °C, and react for 2 h. Suspend the reaction and cool to room temperature. N 2 Add 4-bromoacetophenone 0.5 mmol, 1 ml 1,4-dioxane, 0.5 ml Na t Aqueous OBu solution (5M) was heated to 100°C, and the reaction was continued for 3 h. Stop the reaction and cool to room temperature. The reaction solution was diluted with ethyl acetate, filtered, washed once with water, and the organic phase was washed with Na 2 SO 4 Dry, filter and concentrate. The residue was separated by thin-layer chromatography using ethyl acetate / petroleum ether=1 / 20 as a developing solvent to obtain 91 ...
Embodiment 3
[0035]Take 0.012 mmol of ferroceneimine cyclopalladium-tricyclohexylphosphine adduct, 0.72 mmol of pinacol diboronate, and 1.2 mmol of KOAc into a 10 ml Schlenk tube, and repeatedly vacuumize and fill with N 2 5 times. N 2 Add 0.6 mmol bromobenzene and 2 ml 1,4-dioxane under protection. Stir at room temperature for 5 min, then place in an oil bath heated to 100 °C, and react for 2 h. Suspend the reaction and cool to room temperature. N 2 Add 4-bromoacetophenone 0.5 mmol, 1 ml DMF, 0.5 ml K to the system under atmosphere 3 PO 4 aqueous solution (5M), heated to 100°C, and continued to react for 3 h. Stop the reaction and cool to room temperature. The reaction solution was diluted with ethyl acetate, filtered, washed once with water, and the organic phase was washed with Na 2 SO 4 Dry, filter and concentrate. The residue was separated by thin-layer chromatography using ethyl acetate / petroleum ether=1 / 20 as a developing solvent to obtain 91 mg of the target product with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com