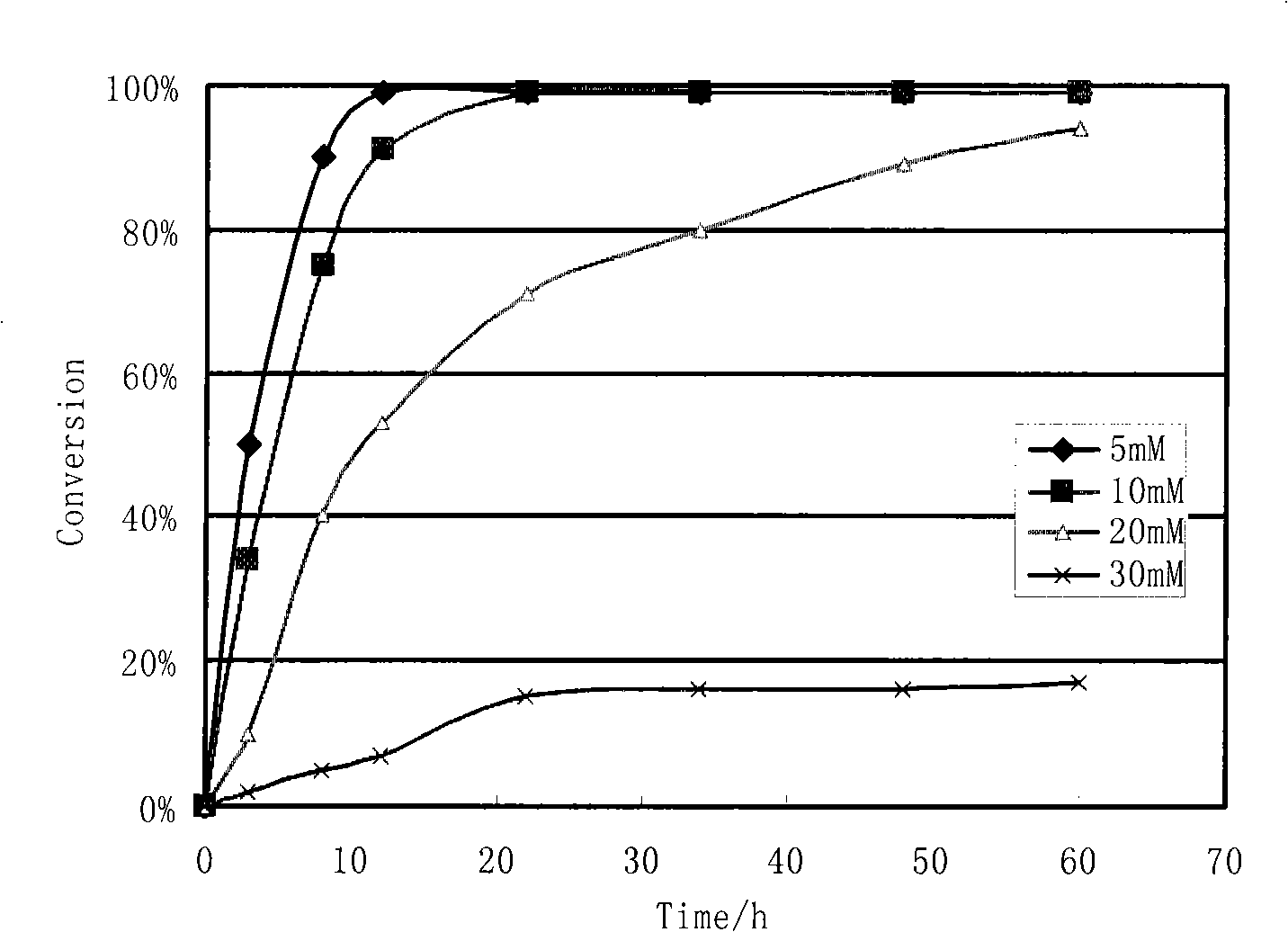
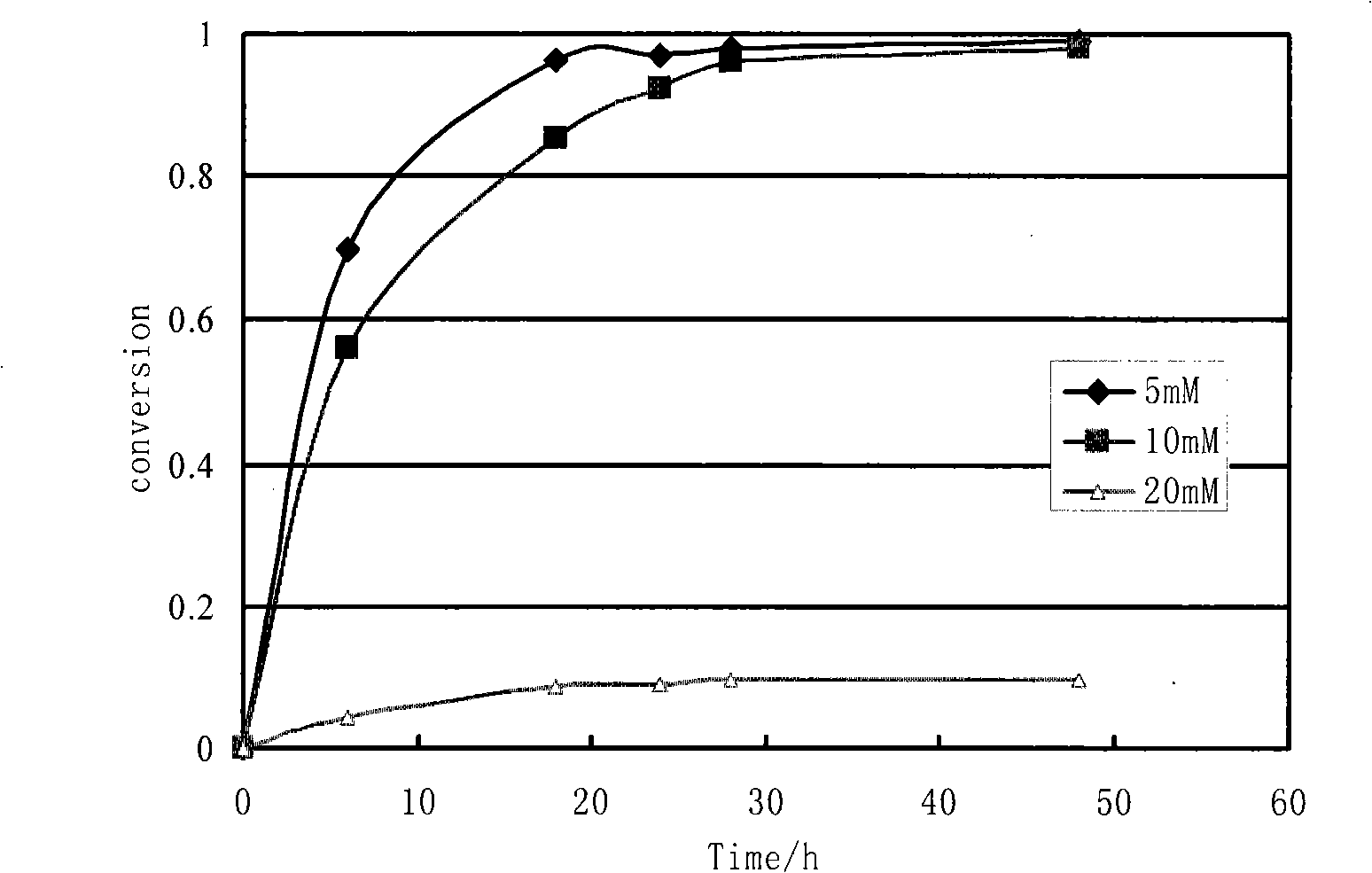
Preparation of two kinds of high optical activity enantiomer of ethyl 4-cyano-3-hydroxybutyrate by biological catalysis method
A technology of ethyl hydroxybutyrate and biocatalysis, applied in biochemical equipment and methods, microorganism-based methods, microorganisms, etc., to achieve the effects of less by-products, high optical purity, and strong reaction specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: plate culture.
[0039] The medium is configured with 1% peptone, 0.5% yeast extract, 1.0% sodium chloride, 1.5% agar, LB agar medium with pH 7.0 (the rest is water), sterilized at 121°C for 20 minutes, cooled, poured plate, respectively Inoculate Bacillus pumilus Phe-C3 and Klebsiella pneumoniaePhe-E4, culture at 28°C for 4-6 days, then transfer to shake flask culture.
Embodiment 2
[0040] Example 2: Shake flask culture.
[0041] Medium configuration Na 2 HPO 4 6.78‰, KH 2 PO 4 3.0‰, NaCl 0.5‰, NH 4 Cl 1.0‰, MgSO 4 0.24‰, CaCl 2 0.01‰, pH 7.4 M9 medium (the rest is water), put it into a Erlenmeyer flask (filling volume 500mL / 1000mL), sterilize at 121°C for 20 minutes, cool, divide into 250mL shake flasks, and add 2% Glucose was inoculated with the above-mentioned Bacillus pumilusPhe-C3 and Klebsiella pneumoniae Phe-E4, respectively, with an inoculation amount of 5-10%, at 28° C., and the rotation speed of the shaker flask was 220 rpm. Cultivate for 2-3 days, centrifuge, collect the bacteria, and save for later use.
Embodiment 3
[0042] Example 3: Fermenter cultivation of Bacillus pumilus Phe-C3.
[0043] The fermenter medium was the same as that used for shake flask culture. When Bacillus pumilusPhe-C3 grew to the late exponential growth stage in the shake flask, it was inoculated into a 5-liter fermenter. The fermenter was prefilled with 2 liters of medium (containing 2% glucose). Fermentation conditions: rotation speed 220rpm, temperature 30°C, air flow rate 1.5L / min. At 20 hours, 50% glucose was added to the fermenter at a rate of 25 g / h to maintain the glucose concentration of the system, while the rotating speed was increased to 400 rpm to increase the ventilation. At 40 hours, reaching its post-exponential growth phase, it was centrifuged at 4°C and stored in a refrigerator until use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com