Preparation method of N-(2-pyridyl/pyrimidyl)indole derivative
A technology for indole derivatives and pyrimidine derivatives, which is applied in the field of preparation of N-indole derivatives, can solve the problems of poor reaction region selectivity, narrow substrate application range, harsh equipment requirements, etc. Processing green, easy post-processing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
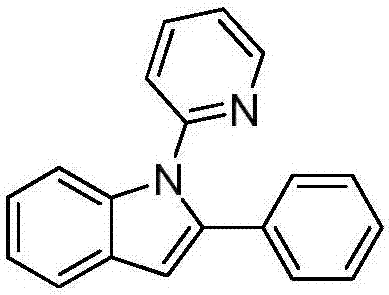
[0020] Preparation of 2-phenyl-1-(2-pyridyl)indole:
[0021]
[0022] Under nitrogen, add 0.2 mmol of N-phenylpyridin-2-amine, 0.3 mmol of (1-alkenyl azido)benzene, 0.01 mmol of palladium trifluoroacetate, 0.4 mmol of potassium persulfate, and 0.08 mmol of triethylenediamine Put it into a reaction tube containing 1mL-1.5mL of toluene, place it in an oil bath at 75°C, and react for 24h. Remove the heat source from the reaction and cool to room temperature. The reaction solution was filtered through celite, concentrated, and purified by column chromatography to obtain 48 mg of the target product with a yield of 89%. The NMR characterization of this compound is as follows: 1 H NMR (400MHz, CDCl 3 )δ8.62 (dd, J=4.9, 1.2Hz, 1H), 7.68 (d, J=4.4Hz, 1H), 7.66 (dd, J=5.0, 1.6Hz, 1H), 7.60 (ddd, J=7.8 , 2.0Hz, 1H), 7.31-7.19(m, 8H), 6.88(d, J=8.0Hz, 1H), 6.80(s, 1H); 13 CNMR (101MHz, CDCl 3 )δ 152.1, 149.2, 139.9, 138.5, 137.7, 132.7, 132.7, 128.7, 128.3, 127.4, 123.0, 122.0, 12...
Embodiment 2
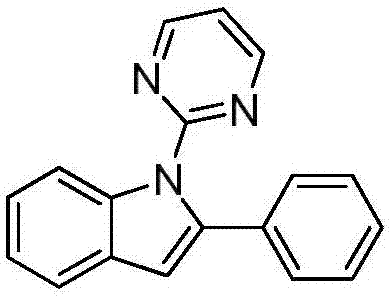
[0024] Preparation of 2-phenyl-1-(2-pyrimidinyl)indole:
[0025]
[0026] Under nitrogen, add 0.2mmol of N-phenylpyrimidin-2-amine, 0.3mmol of (1-alkenyl azido)benzene, 0.01mmol of palladium trifluoroacetate, 0.4mmol of potassium persulfate, and 0.08mmol of triethylenediamine Put it into a reaction tube containing 1mL-1.5mL of toluene, place it in an oil bath at 75°C, and react for 24h. Remove the heat source from the reaction and cool to room temperature. The reaction solution was filtered through celite, concentrated, and purified by column chromatography to obtain 36 mg of the target product with a yield of 66%. The NMR characterization of this compound is as follows: 1 H NMR (400MHz, CDCl 3 )δ8.67(d, J=4.8Hz, 1H), 8.13(d, J=8.1Hz, 1H), 7.65(d, J=7.7Hz, 1H), 7.30-7.22(m, 3H), 7.10( d, J=4.8Hz, 1H), 6.81(s, 1H); 13 C NMR (101MHz, CDCl 3 )δ158.2, 140.5, 138.1, 133.9, 129.3, 129.3, 128.15, 128.1, 127.1, 123.5, 122.1, 120.7, 117.6, 112.8, 108.2.
Embodiment 3
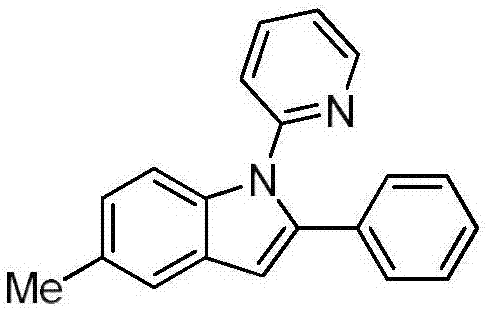
[0028] Preparation of 5-methyl-2-phenyl-1-(2-pyridyl)indole:
[0029]
[0030] Under nitrogen, 0.2mmol of N-(p-tolyl)pyridin-2-amine, 0.3mmol of (1-alkenyl azido)benzene, 0.01mmol of palladium trifluoroacetate, 0.4mmol of potassium persulfate, triethylenediamine 0.08mmol was added to a reaction tube containing 1mL-1.5mL toluene, placed in an oil bath at 75°C, and reacted for 24h. The reaction was removed from the heat source and cooled to room temperature. The reaction solution was filtered through celite, concentrated, and purified by column chromatography to obtain 47 mg of the target product with a yield of 83%. The NMR characterization of this compound is as follows: 1 H NMR (400MHz, CDCl 3 )δ8.58(d, J=4.9Hz, 1H), 7.57(d, J=8.4Hz, 1H), 7.52(dd, J=6.9Hz, 1H), 7.42(s, 1H), 7.24-7.17( m, 4H), 7.12(dd, J=7.4, 4.9Hz, 1H), 7.03(d, J=8.4Hz, 1H), 6.82(d, J=8.0Hz, 1H), 6.71(s, 1H), 2.44(s, 3H); 13 C NMR (101MHz, CDCl 3 )δ152.2, 149.0, 139.9, 137.6, 136.9, 132.8, 130.5, 128...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com