Process for Producing Aliprazo
A technology based on piperazinyl and general formula, which is applied in the field of preparation of aripiprazole, can solve problems such as multiple side reactions, many side reactions, and reduced total yield, and achieve strong reaction specificity, simple post-treatment, and low by-product production. little effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0045] One, the preparation of compound (C): (F)+(E)—→(C)
[0046] 4-[4-(2,3-dichlorophenyl) piperazinyl]-1-butanol (C) can be by 1-(2,3-dichlorophenyl) piperazine (E) and general formula ( A compound of F) is reacted in a solvent at a temperature ranging from room temperature to 200°C, preferably 80-140°C, and the reaction is completed within a few hours to 48 hours. As for the solvent used in this reaction, it can be the following solvents, ether solvents such as tetrahydrofuran, dioxane, ethylene glycol dimethyl ether; aromatic hydrocarbon solvents such as benzene, toluene, pyridine, xylene; alcohol solvents such as Methanol, ethanol, isopropanol; aprotic solvents such as N,N-dimethylformamide, dimethylsulfoxide, hexamethylphosphoramide, acetonitrile. Use of a basic reagent facilitates the reaction. Said alkaline reagent can be potassium hydroxide, potassium carbonate, sodium hydroxide, sodium carbonate, sodium bicarbonate, sodium amide, sodium hydride inorganic base, or ...
example 1
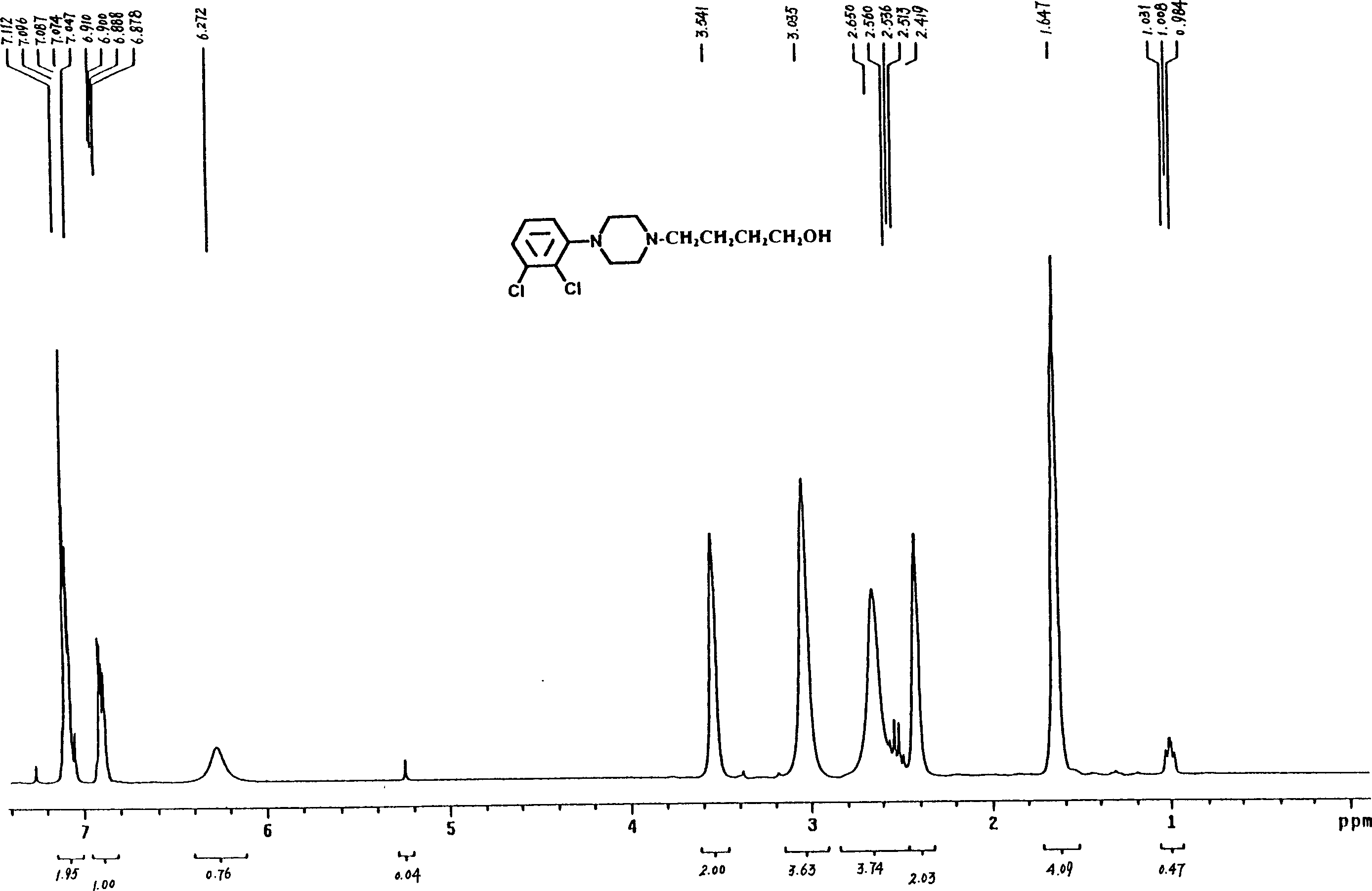
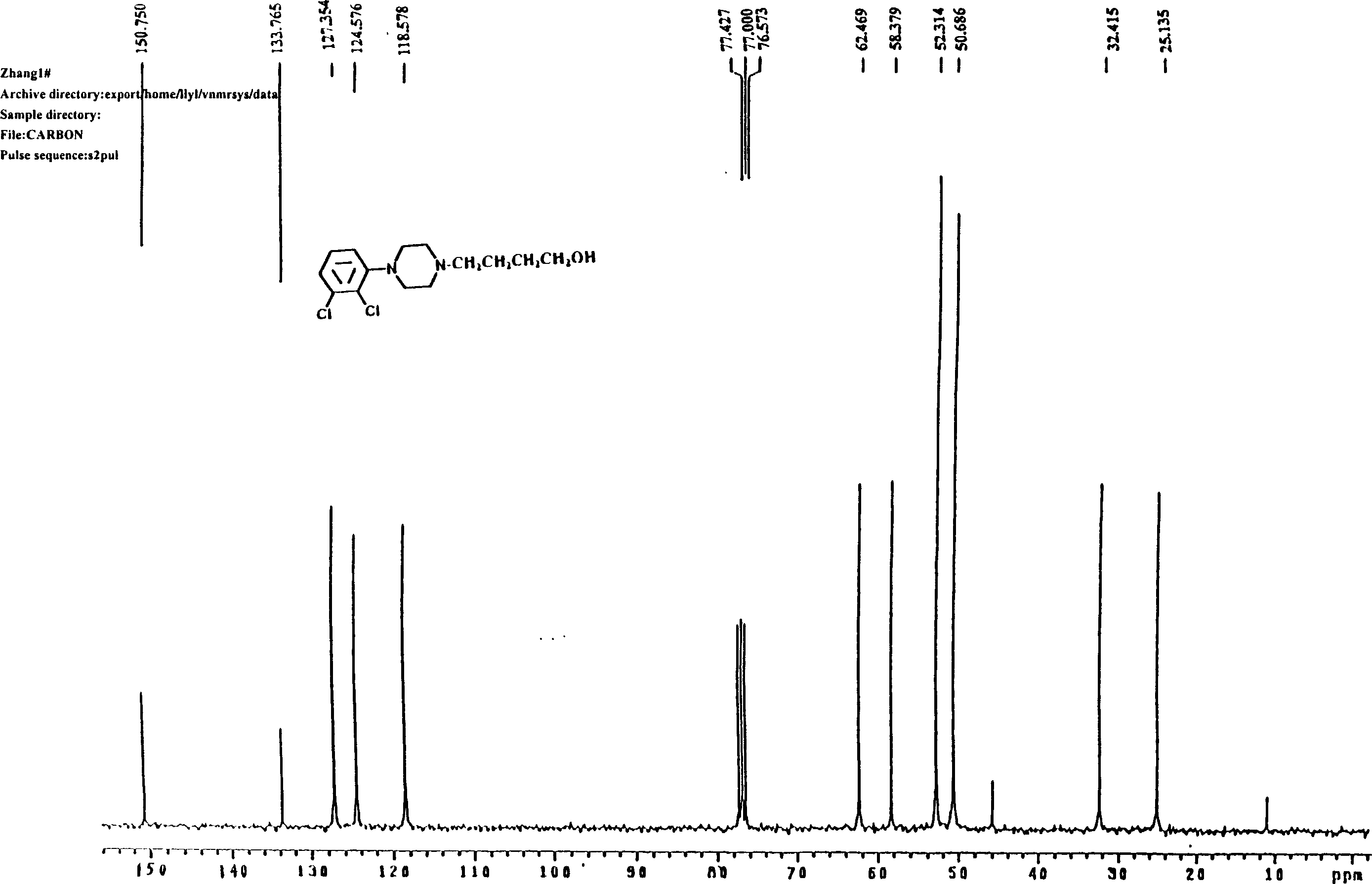
[0055] Preparation of 4-[4-(2,3-dichlorophenyl)piperazinyl]-1-butanol (C)
[0056] Put 5.80g (25mmol) of 1-(2,3-dichlorophenyl)piperazine (E), 3.3g (30mmol) of 4-chloro-1-butanol, and 4.15g (30mmol) of potassium carbonate into the reaction flask successively. ), acetonitrile 25ml, a little potassium iodide, heated and refluxed for 26 hours under stirring, after the reaction was completed, suction filtered, removed inorganic matter, filtered and washed, concentrated the filtrate, recovered part of the organic solvent, added 30ml of ethyl acetate and 10ml of distilled water to the concentrate , separate the organic layer, extract the aqueous layer three times with 10ml ethyl acetate, combine the organic layers, wash with 20% saline until neutral, dry the organic layer with anhydrous sodium sulfate, filter with suction, filter off the desiccant, and recover the organic layer under reduced pressure. solvent. Add 5ml of ethyl acetate to the residue, adjust PH=5 with hydrochloric a...
Embodiment 2-6
[0081] Preparation of 4-[4-(2,3-dichlorophenyl)piperazinyl]-1-butanol (C)
[0082] Dissolve 1-(2,3-dichlorophenyl)piperazine (E) and 4-chloro-1-butanol (F) in the following solvents, respectively use corresponding acid removal agents, heat and keep warm for reaction. After the reaction is completed, it becomes hydrochloride after being processed, and the obtained salt is free into free base (C) after recrystallization, as shown in Table 1:
[0083] Example Solvent Deacidifier Temperature (°C) Time (h) Yield (%)
[0084] 2 Isopropanol Potassium hydroxide 70 30 43.8
[0085] 3 Toluene Triethylamine 100 20 72.5
[0086] 4 THF / DMSO Potassium hydroxide 50 18 69.2
[0087] 5 N,N-Dimethylformamide Potassium carbonate 90 24 61
[0088] 6 Acetonitrile Pyridine 75 30 48.7
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com