Rhodococcus erythropolis and application thereof in microbe-catalyzed preparation of chiral aromatic alcohol
A technology for Rhodococcus erythraea and aromatic alcohol is applied to Rhodococcus erythropolis and its application field in microbial catalyzed preparation of chiral aromatic alcohol, and achieves the effects of high conversion rate, little environmental pollution and strong reaction specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The composition of the fermentation medium of Rhodococcus erythropolis WZ010 (CCTCC NO: M 2011336): peptone 1.0%, yeast extract 0.5%, NaCl 0.5%, solvent is water, pH 7.0-7.2, sterilized at 121°C for 20 min.
[0034] Rhodococcus erythropolis WZ010 was inoculated into 50ml fermentation medium, and cultured for 12 to 48 hours at 30°C and a shaker rotation speed of 200 rpm. After the fermentation broth was centrifuged at 6000 rpm for 15 minutes, the supernatant was discarded, and the cells were washed once with the reaction buffer, and the wet cells obtained were the biocatalyst.
Embodiment 2
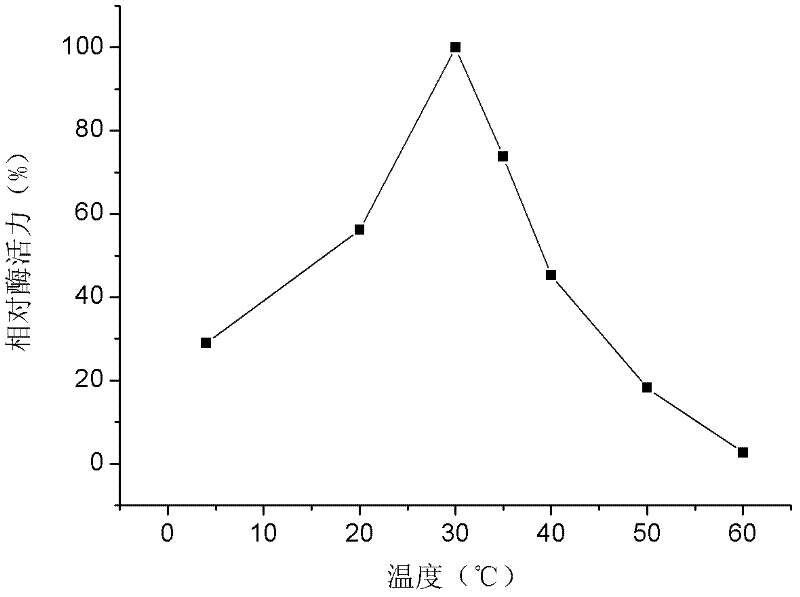
[0036] The 2mL oxidation reaction system contained 50mM glycine-sodium hydroxide buffer (pH10.5), 0.18g Rhodococcus erythropolis wet cell, 40mM mixed 1-phenylethanol. React at different temperatures for 4 hours. After the reaction, the reaction solution was centrifuged at 6000 rpm for 15 min, and 1 mL of the supernatant was mixed with 1 mL of ethyl acetate in equal volumes, and then placed in a shaker for extraction at 30° C. and 200 rpm for 1 to 2 hours. Centrifuge the extract at 6000 rpm for 10 min, take 400 μL of the organic phase, and add excess anhydrous Na 2 SO 4 Dry, perform chiral gas chromatographic analysis of the substrate and its conversion products, see the results figure 1 .
[0037] by figure 1 The results show that Rhodococcus erythropolis WZ010 has catalytic activity for 1-phenylethanol at 4~60℃, and its catalytic activity is highest at 30℃.
Embodiment 3
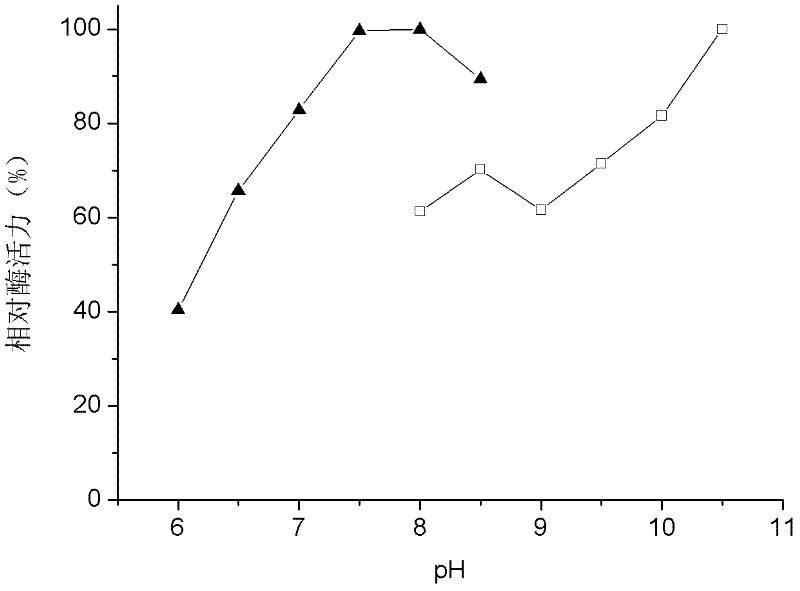
[0039] In a 2mL oxidation reaction system, containing Tris-HCl buffer of pH 8.0~8.8 or glycine-sodium hydroxide buffer (50mM) of pH 8.8~11.0, 0.18g of wet rhodococcus rhodococcus, 40mM mixing 1-Phenylethanol. React at 30°C and different pH for 4 hours. After the reaction, the reaction solution was centrifuged at 6000 rpm for 15 min, and 1 mL of the supernatant was mixed with 1 mL of ethyl acetate in equal volumes, and then placed in a shaker for extraction at 30° C. and 200 rpm for 1 to 2 hours. Centrifuge the extract at 6000 rpm for 10 min, take 400 μL of the organic phase, and add excess anhydrous Na 2 SO 4 Dry, perform chiral gas chromatographic analysis of the substrate and its conversion products, see the results figure 2 .
[0040] by figure 2 The results show that Rhodococcus erythropolis WZ010 has activity between pH 8 and 11, and its catalytic activity increases with the increase of pH.
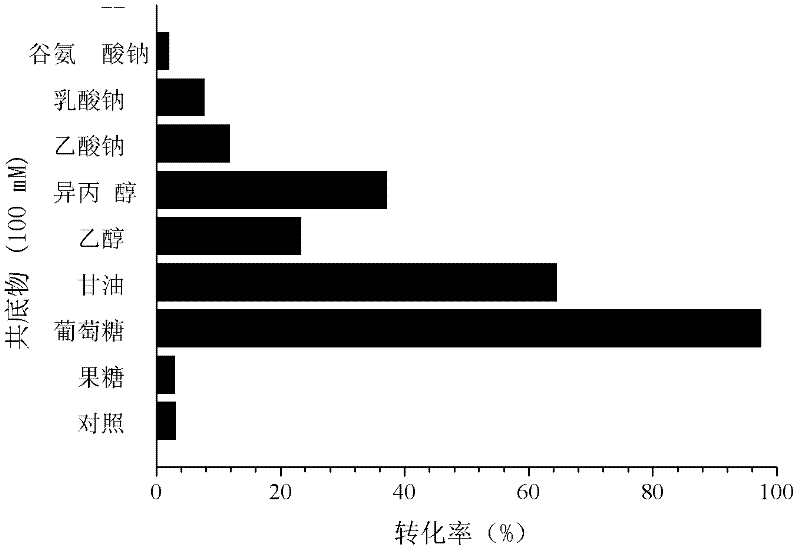
[0041] In the 2mL reduction reaction system, respectively containing pH 6.0~7.0 d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com