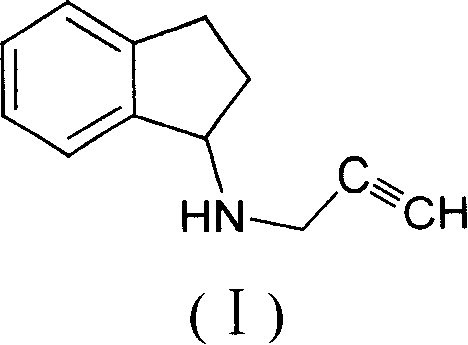
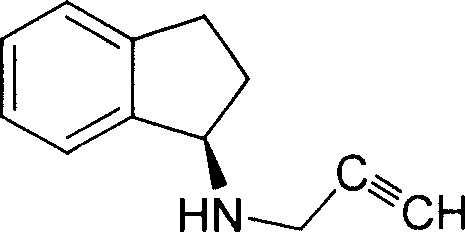
Simple and novel process for preparing indenes derivatives
A derivative and novel technology, applied in the field of preparation of anti-Parkinson's disease drug rasagiline, which can solve the problems of long synthetic route, reduced overall yield, and increased cost of the final product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] The synthesis of embodiment 1 1-propargyl indene amine
[0018] Add 130 ml of absolute ethanol to a 250 ml three-necked flask, add 13.2 g of 1-indanone and 6.6 g of propargylamine to it. At 0°C, 3.8 g of sodium borohydride was added in portions. After the addition was completed, the reaction was continued by heating to 45°C, and the reaction was stopped after about 24 hours. Concentrate to remove ethanol, add 130 ml of pure water, extract three times with 130 ml of ethyl acetate, combine the ethyl acetate phases, dry with anhydrous magnesium sulfate, filter with suction, concentrate to remove ethyl acetate to obtain 12.5 g of the target compound.
Embodiment 2
[0019] The synthesis of embodiment 2 1-propargyl indene amines
[0020] Add 100 ml of absolute ethanol to a 250 ml three-necked flask, add 10 g of 1-indanone and 5 g of propargylamine to it. At 0°C, 4 g of sodium borohydride was added in portions. After the addition was completed, the reaction was continued by heating to 45°C, and the reaction was stopped after about 24 hours. Concentrate to remove ethanol, add 100 ml of pure water, extract three times with 100 ml of ethyl acetate, combine the ethyl acetate phases, dry over anhydrous magnesium sulfate, filter with suction, concentrate to remove ethyl acetate to obtain 9.2 g of the target compound.
Embodiment 3
[0021] The synthesis of embodiment 3 1-propargyl indene amines
[0022] Add 150 milliliters of absolute ethanol to the high-pressure reaction tank, add 15 grams of 1-indanone and 7.5 grams of propargylamine, and add 1 g of palladium carbon (5%). Catalytic hydrogenation was carried out at 45°C and 4 atmospheres, and the reaction was stopped after about 24 hours. Remove the catalyst by suction filtration under reduced pressure, concentrate to remove ethanol, add 150 ml of pure water, extract three times with 150 ml of ethyl acetate, combine the ethyl acetate phases, dry with anhydrous magnesium sulfate, filter with suction, and concentrate to remove ethyl acetate to obtain the target compound 13.4 grams.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com