Long-acting sustained-release preparation for resisting Parkinson's disease and preparation method thereof
A slow-release preparation, Parkinson's disease technology, applied in the directions of drug combination, drug delivery, pharmaceutical formulation, etc., can solve the problems of prescription compatibility, product burst release, increase prescription complexity, etc., achieve delayed release period, reduce The effect of fluctuations in concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
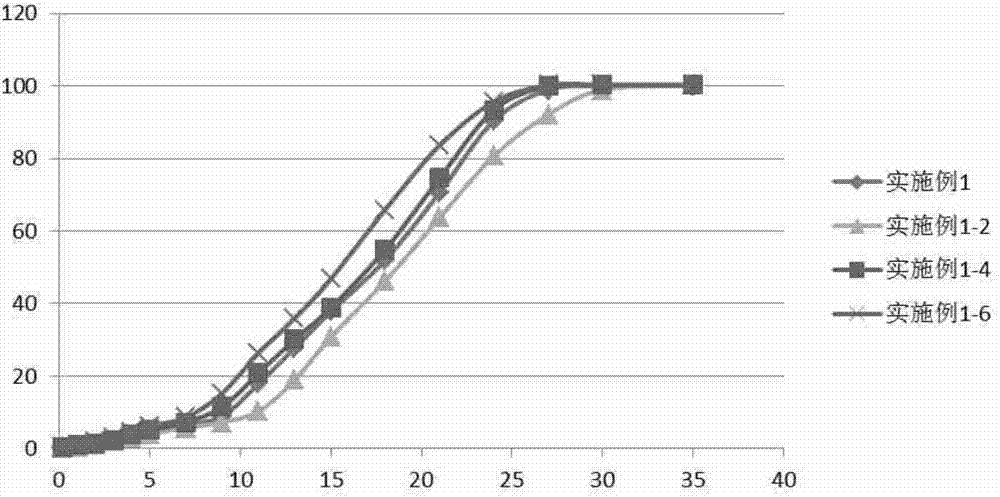
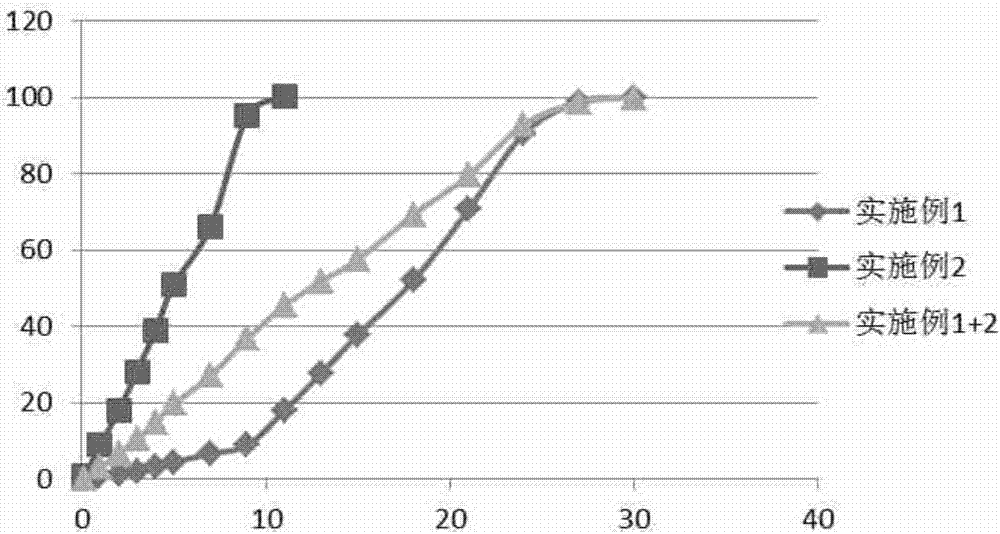
Embodiment 1
[0058] Example 1. Preparation of sustained-release microspheres.
[0059] Dissolve 8.00g PLGA (lactide / glycolide=65 / 35, hereinafter the same, all are lactide / glycolide, 0.65dl / g, 71kDa, ester end group) in 80.00g dichloromethane, get Homogeneous solution A; dissolve 2.00 g of rasagiline in 10.00 g of ethanol to obtain homogeneous solution B; under the vortex condition of 3000 rpm, add solution A to solution B to obtain homogeneous emulsion C. The homogeneous solution C was added into 1.0% polyvinyl alcohol aqueous solution at 4° C., and stirred with a mechanical stirrer at 2000 rpm to prepare microspheres. Then the temperature was raised to 40° C. to volatilize the organic solvent, and then the microspheres were filtered, washed with water for injection for 7 times, and then freeze-dried to obtain sustained-release microspheres.
Embodiment 2
[0060] Example 2. Preparation of sustained-release microspheres.
[0061] Dissolve 8.00g PLGA (65 / 35, 0.65dl / g, 71kDa, ester end group) in 80.00g dichloromethane to obtain a homogeneous solution A; dissolve 2.00g rasagiline in 10.00g ethanol to obtain a homogeneous solution B: Under ultrasonic conditions, with a power of 300w, add solution A to solution B to obtain a homogeneous emulsion C. The homogeneous solution C was added into the 1.0% polyvinyl alcohol continuous aqueous phase at 4° C., and the microspheres were homogeneously prepared under high pressure under a pressure of 900 bar. Then the temperature was raised to 40°C to volatilize the organic solvent, and then the microspheres were filtered to remove the continuous water phase, and the microspheres were washed 7 times with water for injection, and then freeze-dried to obtain sustained-release microspheres.
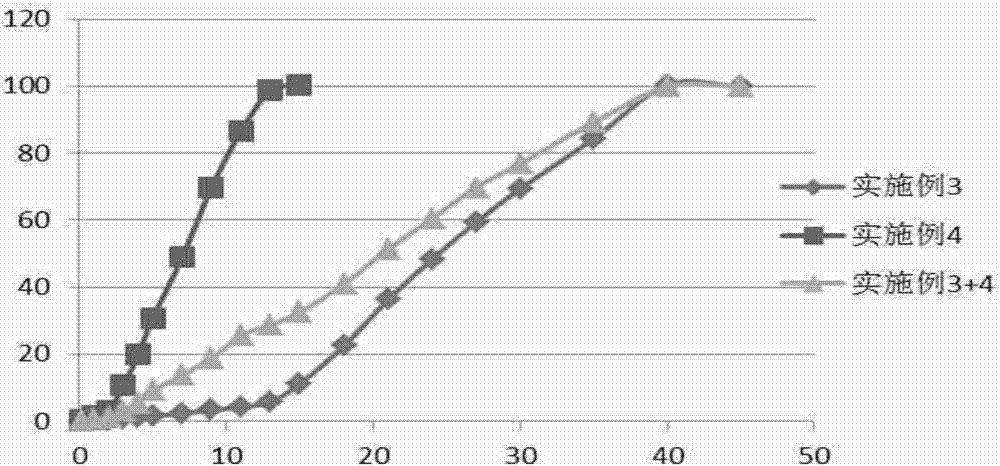
Embodiment 3
[0062] Example 3. Preparation of sustained release microspheres.
[0063] Dissolve 7.00g PLGA (75 / 25, 0.5dl / g, 57kDa, carboxyl end group) in 70.00g ethyl acetate to obtain homogeneous solution A; dissolve 3.00g rasagiline in 15.00g acetic acid to obtain homogeneous solution B ; Under the vortex condition of 3000rpm, solution A was added to solution B to obtain uniform emulsion C. The homogeneous solution C was added into the 0.5% polyvinyl alcohol continuous aqueous phase at 4°C, and a 4000rpm homogenizer was used to homogeneously prepare microspheres. Then the temperature was raised to 40° C. to volatilize the organic solvent, and then the microspheres were filtered, washed with water for injection for 7 times, and then freeze-dried to obtain sustained-release microspheres.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com