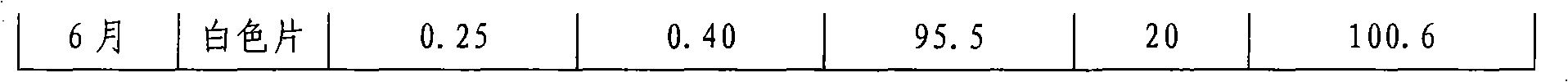Orally disintegrating tablet of Rasagiline or medicine salts thereof and preparation method thereof
A technology of orally disintegrating tablets and medicinal salts, applied in the field of medicine, can solve problems such as discomfort, lack of suitable products and preparation methods, and limited applications, and achieve the effect of stable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] The composition of rasagiline or its medicinal salt orally disintegrating tablets is as follows:
[0050] Raw materials
mg / tablet
% / piece
Rasagiline mesylate
0.25
0.36
62.4
89.14
2.8
4
1.4
2
peppermint
1.4
2
Micropowder silica gel
0.35
0.5
[0051] Raw materials
mg / tablet
% / piece
1.4
2
Sheet weight
70
100
[0052] Grind the rasagiline mesylate raw material, pass through a 100-mesh sieve, and set aside. After taking the prescribed amount of rasagiline mesylate and mixing it evenly with each auxiliary material, press it into a circular flat tablet with a diameter of 6 mm.
[0053] test results:
[0054] A. Hardness measurement (YD-1 tablet hardness tester) result: average value 3.5±0.5Kg (n=10).
[0055] B...
Embodiment 2
[0059] The composition of rasagiline or its medicinal salt orally disintegrating tablets is as follows:
[0060] Raw materials
mg / tablet
% / piece
Rasagiline
0.5
0.5
91
91
2
2
4
4
Micropowder silica gel
2
2
0.5
0.5
Sheet weight
100
100
[0061] After mixing the raw materials evenly, press them into circular biconvex tablets with a diameter of 7 mm.
[0062] The average value of hardness is 3.5±0.5Kg (n=10).
[0063] Determination of disintegration time limit: 5-15 seconds (n=6)
Embodiment 3
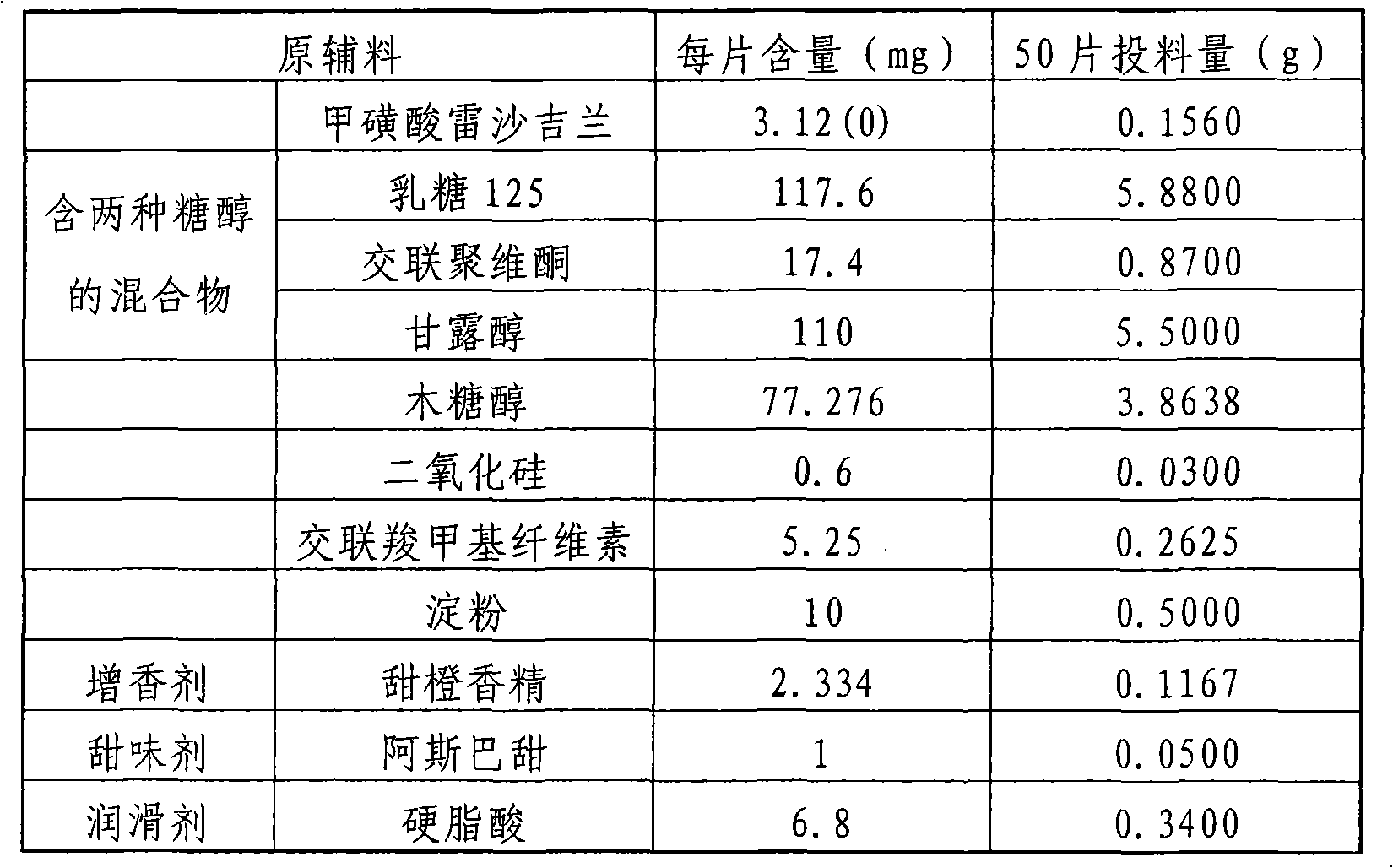
[0065] The composition of rasagiline or its medicinal salt orally disintegrating tablets is as follows:
[0066] Raw materials
mg / tablet
% / piece
Rasagiline mesylate
1
0.74
117.8
87.26
4.05
3
6.75
5
Micropowder silica gel
2.7
2
2.7
2
Sheet weight
135
100
[0067] After the components of the raw materials are mixed evenly, they are pressed into a circular double-sided concave tablet with a diameter of 8 mm.
[0068] The average value of hardness is 3±0.5Kg (n=10).
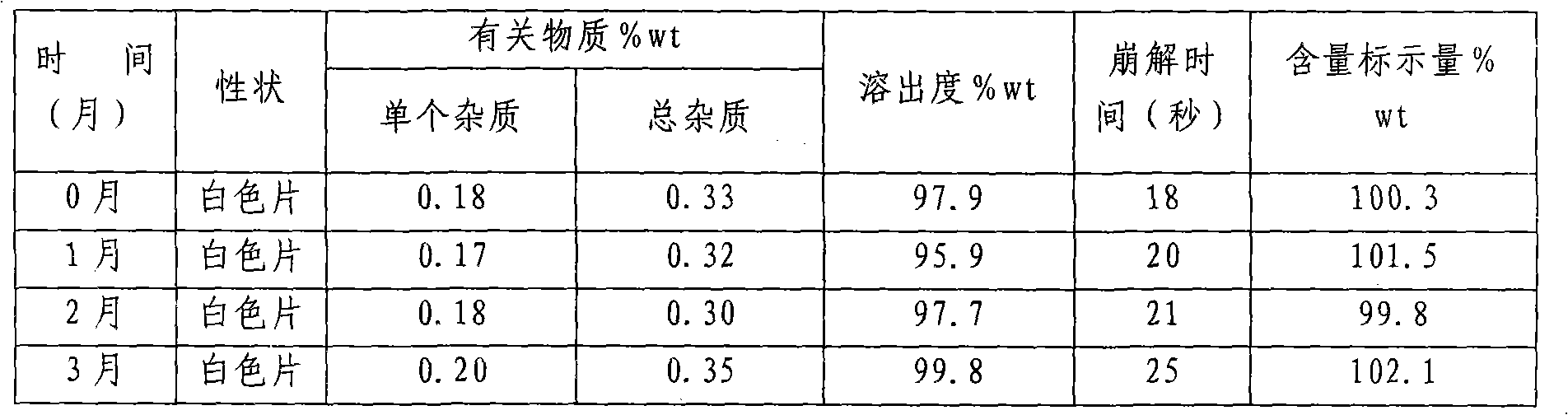
[0069] Determination of disintegration time limit: 15-25 seconds (n=6)
[0070] The friability is less than 0.5%.
[0071] Dissolution: more than 95% dissolved in 10 minutes (n=6).
[0072] Content uniformity: in line with the regulations.
[0073] Accelerated test for 6 months, all quality...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| friability | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com