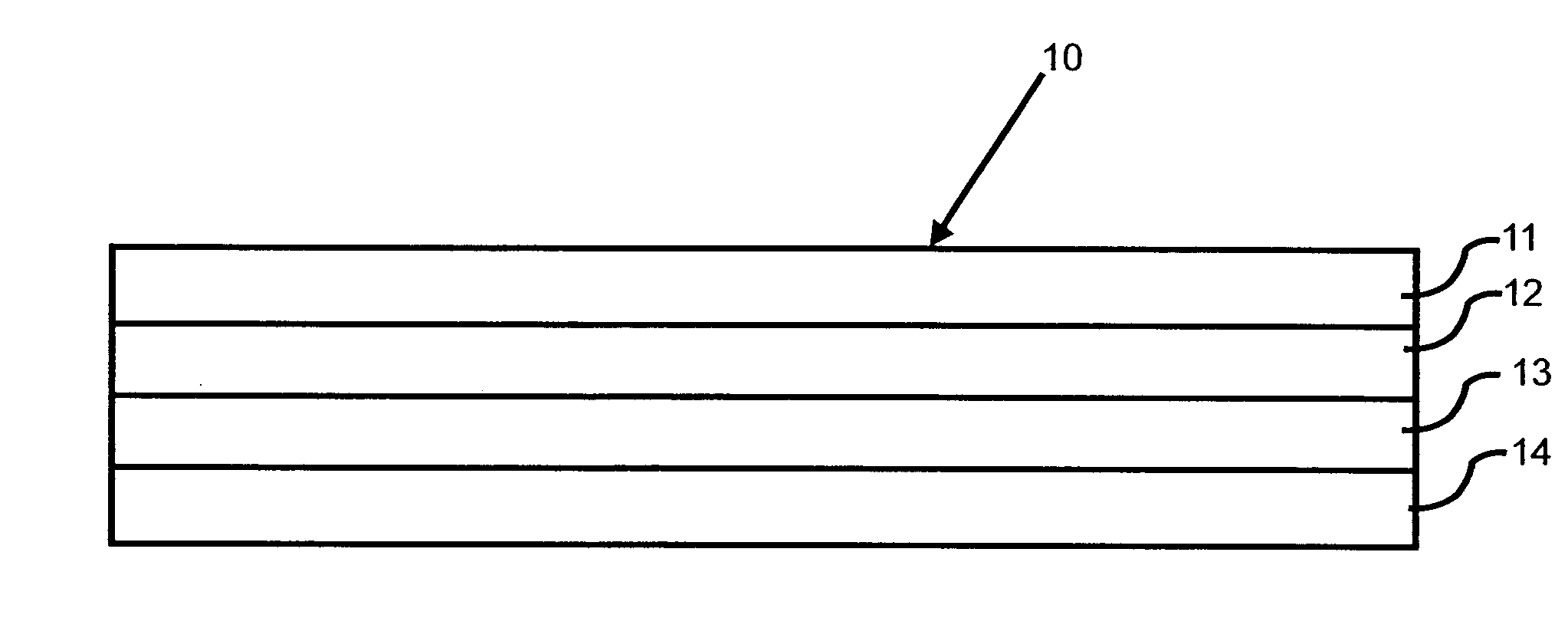
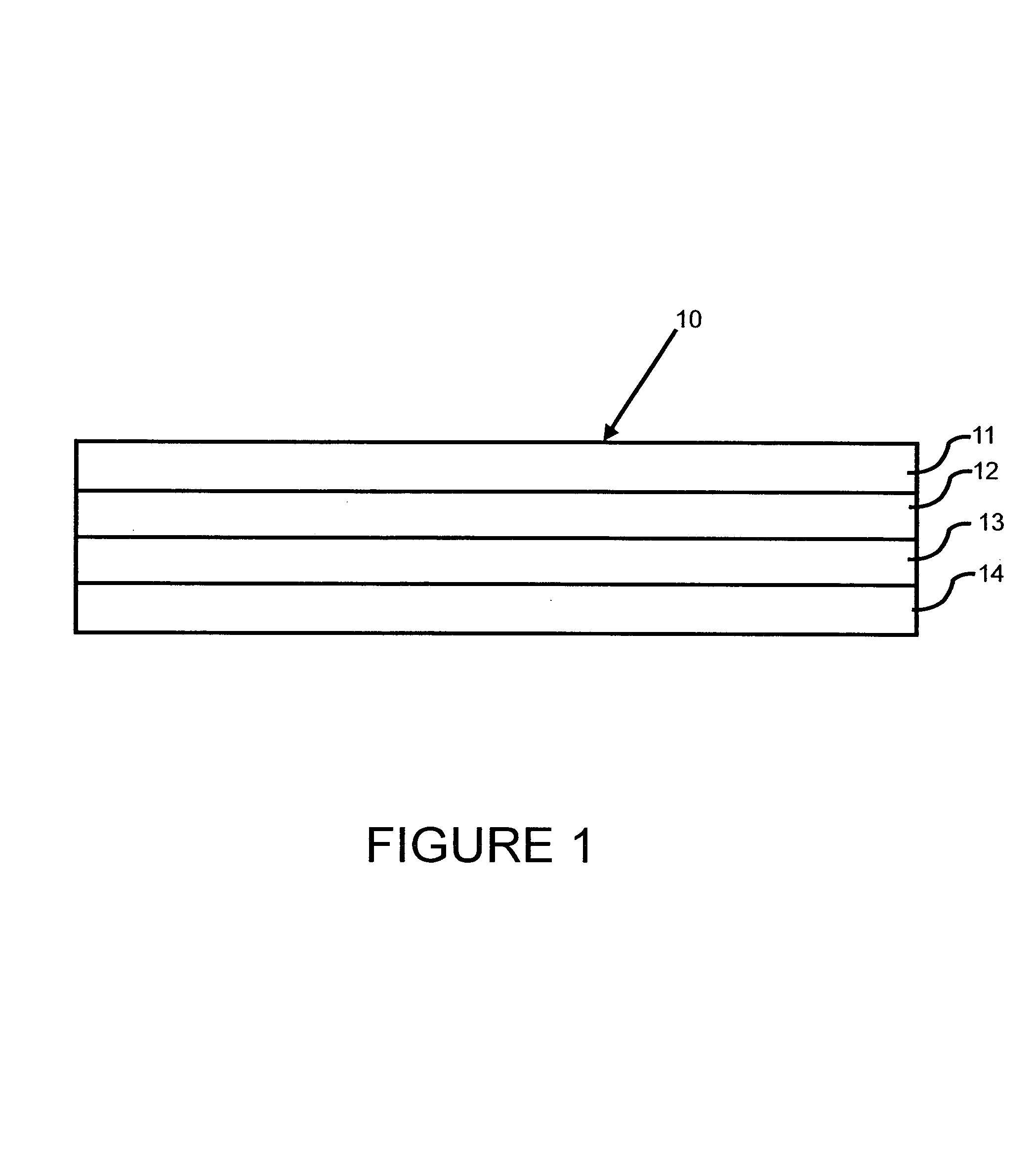
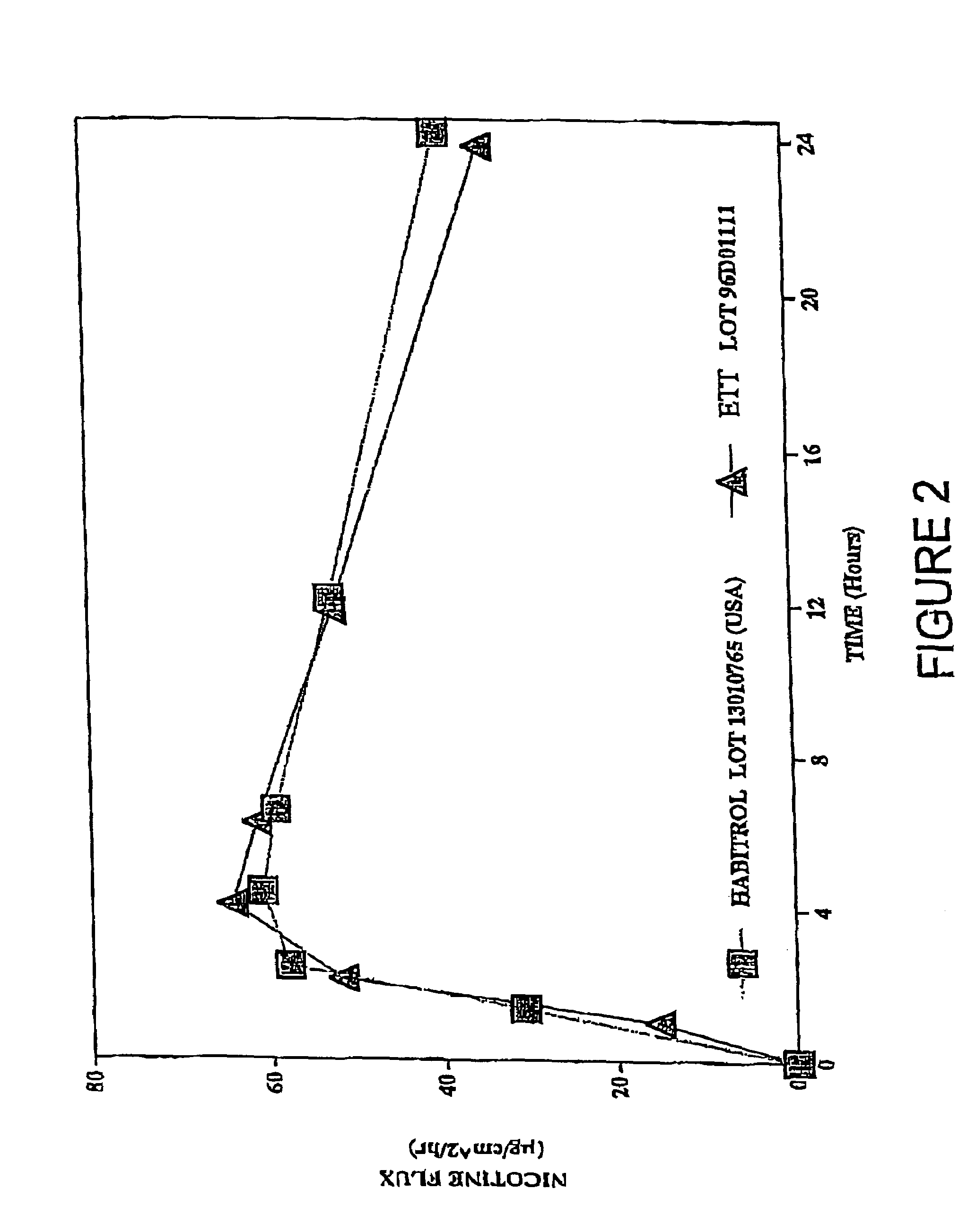
Transdermal patch for delivering volatile liquid drugs
a technology of volatile liquid drugs and patches, which is applied in the direction of bands, dressings, organic active ingredients, etc., can solve the problems that the prior art does not teach or suggest the transdermal administration of mecamylamine itself to treat nicotine dependency, and the patent does not teach
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation and Testing of Nicotine Patch
[0035]Nicotine was added to a hexane solution of Dow Corning BIO-PSA amine-compatible silicone pressure sensitive adhesive to a level of approximately 12% by weight based on the combined dry weight of adhesive and nicotine. The resulting hexane solution of adhesive and nicotine was coated onto a 3M Scotchpak 1109 polyester / polyolefin backing at 13.8 mg / cm2 (1.63 mg / cm2 nicotine and 12.17 mg / cm2 adhesive) and the coated backing was dried at 30° C. to 40° C. for about 3 min.
[0036]National Starch DuroTak 87 2194 acrylic solution pressure sensitive adhesive was coated onto a 125 micron thick Daubert Coater Products 1-5 PESTR (Matte)-164Z siliconized polyester release liner at 13.18 mg / cm2 and the coated release liner was dried at 100° C. for about 10 min.
[0037]The dried silicone adhesive / nicotine-coated backing layer subassembly was then laminated to the dried acrylic adhesive-coated release liner subassembly to form a four-layer laminated compos...
example 2
Preparation and Testing of Nicotine / Mecamylamine Patches
[0040]Nicotine and mecamylamine were added to a hexane solution of Dow Corning BIO-PSA amine compatible silicone pressure sensitive adhesive. Two batches were made: one contained approximately 10% nicotine and 6.4% mecamylamine based on the total dry weight of the adhesive and the two drugs; and a second contained approximately 10% nicotine and 4.2% mecamylamine, based on the total dry weight of the adhesive and the two drugs. The batches were separately coated onto a 3M Scotchpak 1109 polyester / polyoefin backing film at 9.6 mg / cm2 (0.96 mg / cm2 nicotine, 0.61 mg / cm2 mecamylamine, 8.03 mg / cm2 adhesive for the first batch; 0.96 mg / cm2 nicotine, 0.40 mg / cm2 mecamylamine, 8.24 mg / cm2 adhesive for the second batch) and then dried at 30–40° C. for about 2 min.
[0041]A blend of two National Starch DuroTak acrylic solution polymers, 87-2196 and 87-2516, 25% and 75% w / w, respectively, was made. The blend was coated at 8.0 mg / cm2 onto a 7...
example 3
Preparation and Testing of Selegiline Patch
[0044]Selegiline was added to a hexane solution of Dow Corning BIO-PSA® amine-compatible silicone pressure sensitive adhesive to a concentration of approximately 10% by weight based on the combined dry weight of adhesive and selegiline. The resulting hexane solution of adhesive and selegiline was coated on 3M Scotchpak 1109 polyester / polyolefin backing at 10.0 mg / cm2 (1.0 mg / cm2 selegiline and 9.0 mg / cm2 adhesive) and the coated backing was dried at 300 C to 400 C for about 3 minutes.
[0045]National Starch DuroTak® 87-2194 acrylic solution pressure sensitive adhesive was coated onto a 125 micron thick Daubert Coated Products 1-5 PESTER (Matte)-164Z siliconized polyester release liner at 8.0 mg / cm2 and the coated release liner was dried at about 1000 C for about 10 minutes.
[0046]The dried silicone adhesive / selegiline-coated backing layer subassembly was then laminated to the dried acrylic adhesive-coated release liner subassembly to form a fo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com