Improved preparation method of nicergoline
A technique for nicergoline and ergoline, applied in the field of improved preparation of nicergoline, can solve the problems of high activity of 5-bromonicotinyl chloride, difficult recycling of organic bases, unfavorable industrialized production, etc., achieves good impurity removal effect, labor The effect of reduced strength and easy recycling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
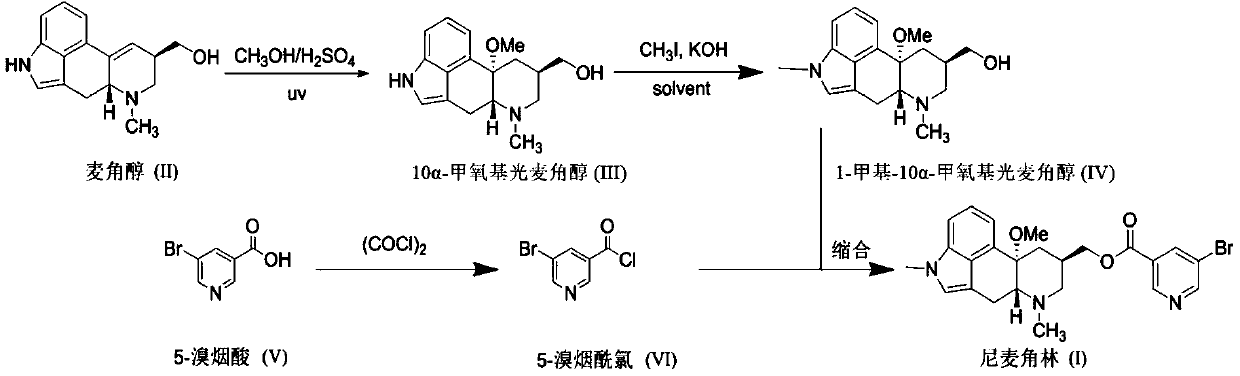
[0047] Add ergot alcohol (50.8g, 0.2mol) into the mixed solution of 98% sulfuric acid (20.0g, 0.2mol) and anhydrous methanol (500mL) prepared in advance, turn on the ultraviolet lamp irradiation (wavelength 254nm, power 125W), control the internal temperature Stir and react at 30-40°C for about 48 hours, TLC detection (UV254, methanol / chloroform / ammonia = 1:8:0.1) the reaction is complete. Add pre-cooled sodium methoxide-methanol (0.4mol) dropwise to the reaction solution, control the rate of addition so that the internal temperature of the reaction solution does not exceed 10°C, after the addition is complete, remove the sodium sulfate generated by filtration, and decolorize the mother liquor with activated carbon (10-15%) , filtered, concentrated under reduced pressure to recover methanol, and the residue was recrystallized with acetonitrile to obtain 49.7 g of 10α-methoxy phoergodol, with a HPLC purity of 95.1%.
[0048] 10α-methoxy phoergodol (42.9g, 0.15mol) was dissolved...
Embodiment 2
[0051] Add ergot alcohol (50.8g, 0.2mol) into pre-configured 98% sulfuric acid (60.0g, 0.6mol) mixed solution of anhydrous methanol (750mL), cool the reaction solution to about 20°C, turn on the ultraviolet lamp irradiation (wavelength 365nm, power 250W), control the internal temperature at 20-30°C and stir the reaction for about 24 hours, TLC detection (UV254, methanol / chloroform / ammonia=1:8:0.1) the reaction is complete. Cool the reaction liquid to 0-5°C, add pre-cooled sodium hydroxide-methanol (1.2mol) dropwise to the reaction liquid while stirring, control the drop rate so that the internal temperature of the reaction liquid does not exceed 20°C, after the addition is complete, filter to remove the sulfuric acid formed Sodium, the mother liquor is decolorized with activated carbon (10-15%), filtered, concentrated under reduced pressure to recover methanol, the residue is slurried with acetonitrile-methanol (10:1, V / V), filtered, and dried to obtain 10α-methoxyphotoergot A...
Embodiment 3
[0055] Add ergot alcohol (50.8g, 0.2mol) into the mixed solution of 98% sulfuric acid (100g, 1.0mol) and anhydrous methanol (750mL) prepared in advance, cool the reaction solution to about 10°C, turn on the ultraviolet lamp irradiation (wavelength 365nm , power 500W), control the temperature at 10-20°C and stir the reaction for about 9 hours, TLC detection (UV254, methanol / chloroform / ammonia = 1:8:0.1) the reaction is complete. Cool the reaction liquid to 0-5°C, add pre-cooled sodium hydroxide-methanol (2.0mol) dropwise to the reaction liquid while stirring, control the drop rate so that the internal temperature of the reaction liquid does not exceed 20°C, after the addition is complete, filter to remove the sulfuric acid formed Sodium, the mother liquor is decolorized with activated carbon (10-15%), filtered, concentrated under reduced pressure to recover most of the methanol, and the residue is recrystallized with acetonitrile-methanol (5:1, V / V) to obtain 10α-methoxy glaucol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com