Nicergoline lyophilized preparation with excellent stability
A kind of technology of nicergoline and excipient, applied in the composition of new nicergoline freeze-dried preparation and its production field, can solve problems such as gap, absolute bioavailability less than 5%, complex process or composition, and avoid harm , Stability improvement, Stability improvement effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
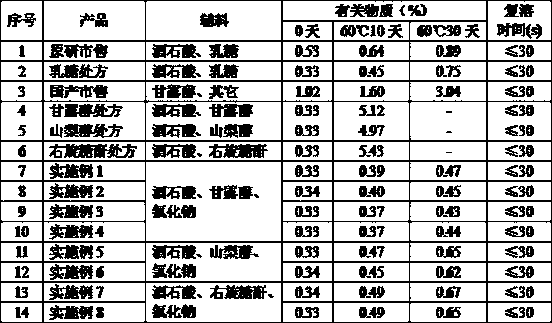
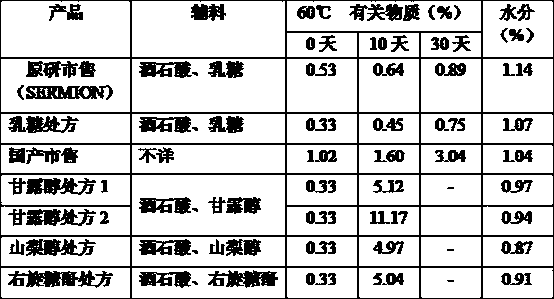
[0032] A kind of Nicergoline freeze-dried powder injection for injection, it is made up of following components: Nicergoline 2g, tartaric acid 0.5g, mannitol 20g, sodium chloride 0.5g. Dissolve tartaric acid, mannitol, and sodium chloride in about 800g of water for injection (20-25°C), stir until dissolved, add Nicergoline and continue stirring until it is completely dissolved, make up water for injection to 1000g, and check that the pH value should be 3.0 -5.0. After being filtered by a 0.22 micron microporous membrane, it is filled in a vial, half-tightened, and placed in a freeze dryer for freeze-drying in the following specific ways:
[0033] Pre-freezing: reduce the temperature of the shelf to -40°C in 2 hours and maintain it for 2 hours.
[0034] The first stage of drying: After pre-freezing, vacuumize, the vacuum degree is controlled at ≤10pa, the temperature of the partition is raised to -10°C in 1 hour, and maintained for 6 hours, and the temperature of the partition...
Embodiment 2
[0039] A kind of Nicergoline freeze-dried powder injection for injection, it is made up of following components: Nicergoline 2g, tartaric acid 0.5g, mannitol 20g, sodium chloride 2g. Dissolve tartaric acid, mannitol, and sodium chloride in about 400g of water for injection (25-30°C), stir until dissolved, add Nicergoline and continue stirring until it is completely dissolved, make up water for injection to 1000g, and check that the pH value should be 3.0 -5.0. After being filtered by a 0.22 micron microporous membrane, it is filled in a vial, half-tightened, and placed in a freeze dryer for freeze-drying in the following specific ways:
[0040] Pre-freezing: reduce the temperature of the shelf to -40°C in 1 hour and maintain it for 2 hours.
[0041] The first stage of drying: After pre-freezing, vacuumize, the vacuum degree is controlled at ≤10pa, the temperature of the partition is raised to -10°C in 1 hour, and maintained for 4 hours, and the temperature of the partition is...
Embodiment 3
[0046] A kind of Nicergoline freeze-dried powder for injection, it is made up of following components: Nicergoline 4g, tartaric acid 1g, mannitol 40g, sodium chloride 4g. Dissolve tartaric acid, mannitol, and sodium chloride in about 600g of water for injection (15-20°C), stir until dissolved, add Nicergoline and continue stirring until it is completely dissolved, make up water for injection to 1000g, and check that the Ph value should be 3.0 -5.0. After being filtered by a 0.22 micron microporous membrane, it is filled in a vial, half-tightened, and placed in a freeze dryer for freeze-drying in the following specific ways:
[0047]Pre-freezing: reduce the temperature of the shelf to -50°C in 2 hours and maintain it for 2 hours.
[0048] The first stage of drying: After pre-freezing, vacuumize, the vacuum degree is controlled at ≤10pa, the temperature of the partition is raised to -15°C in 1 hour, and maintained for 10 hours, and the temperature of the partition is raised to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com