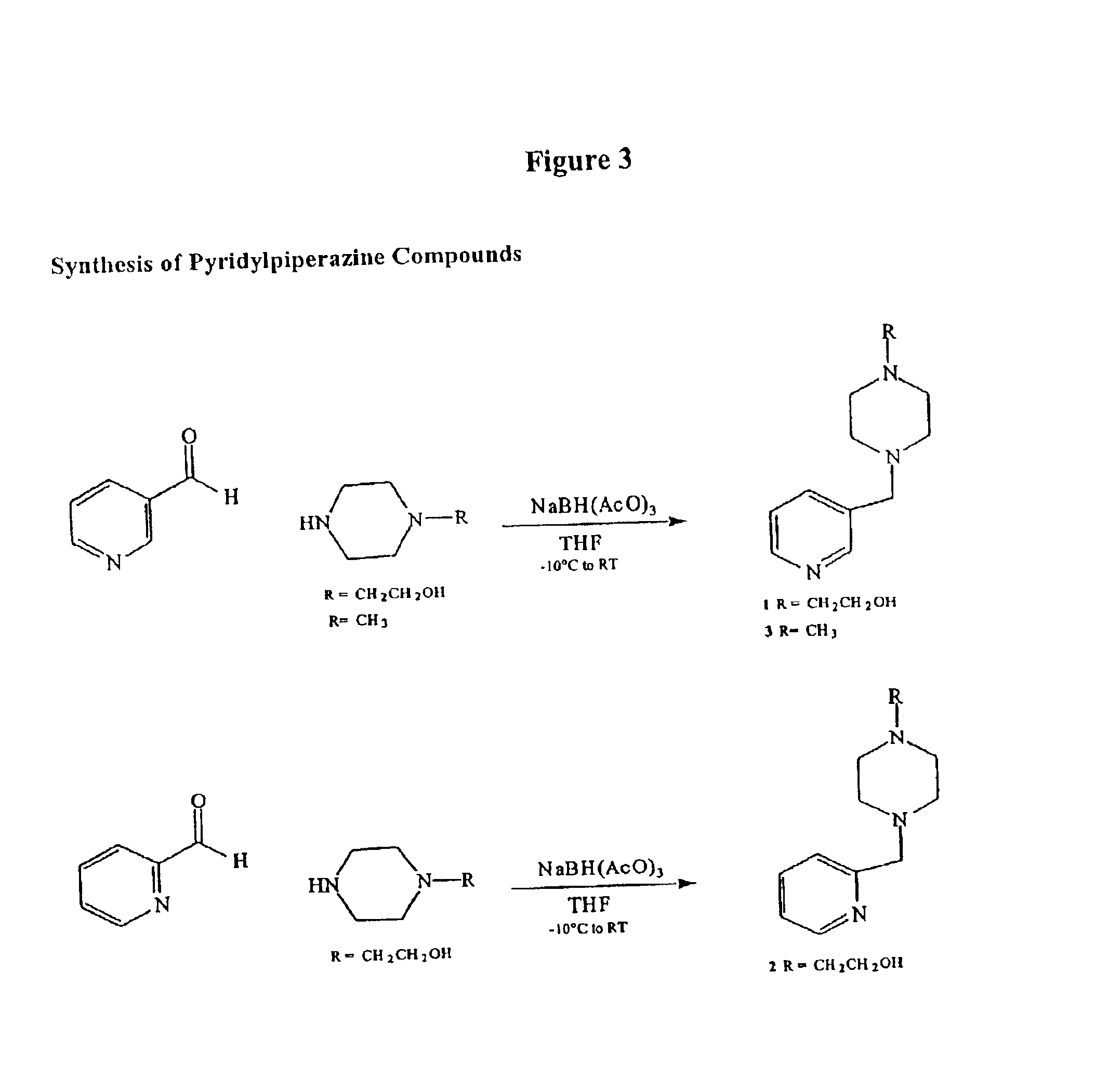
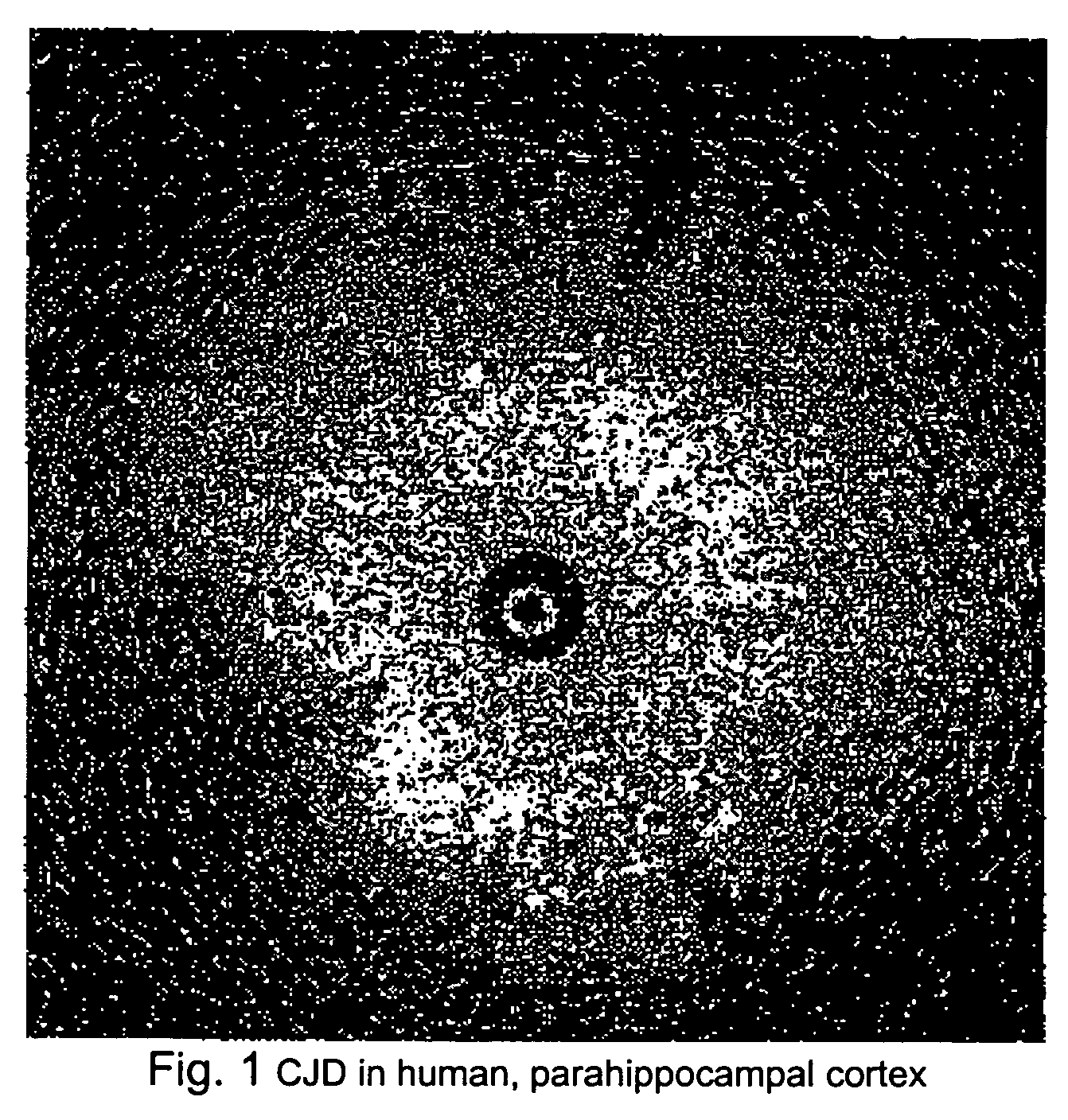
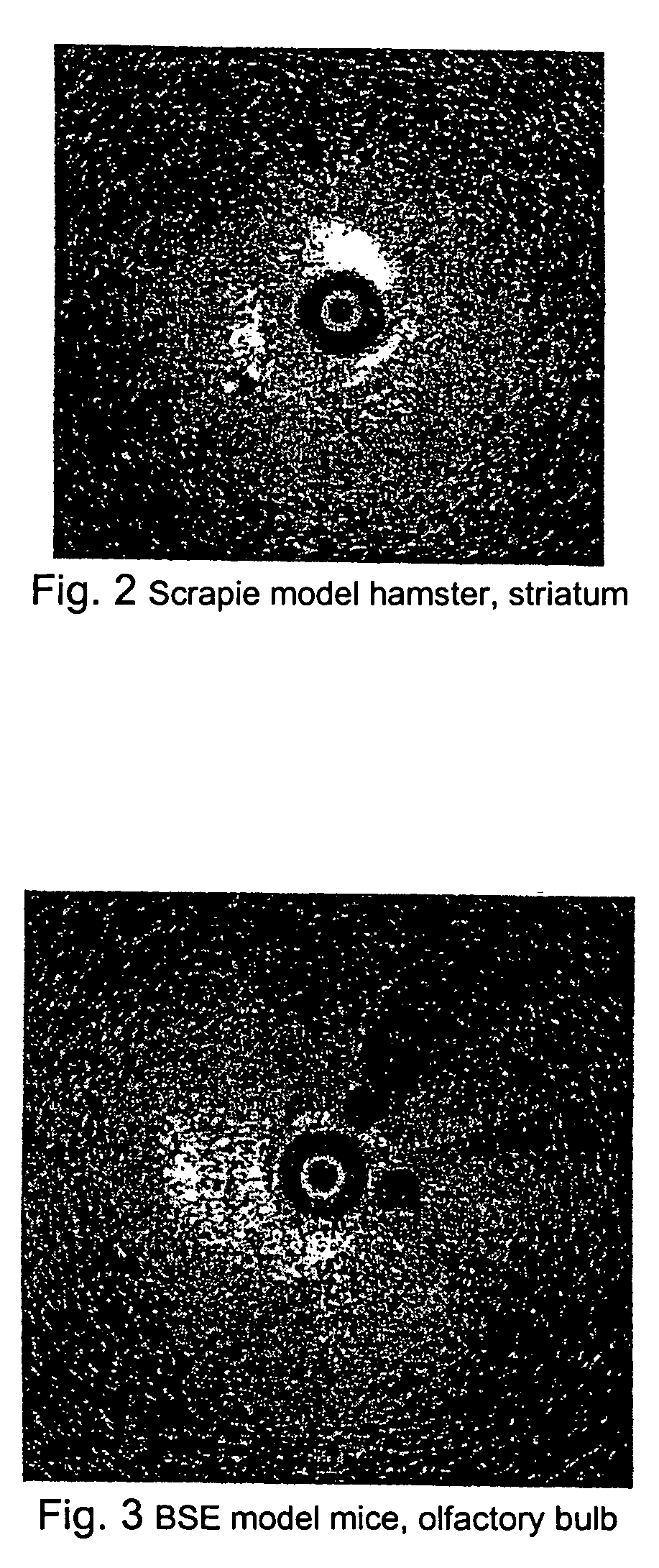
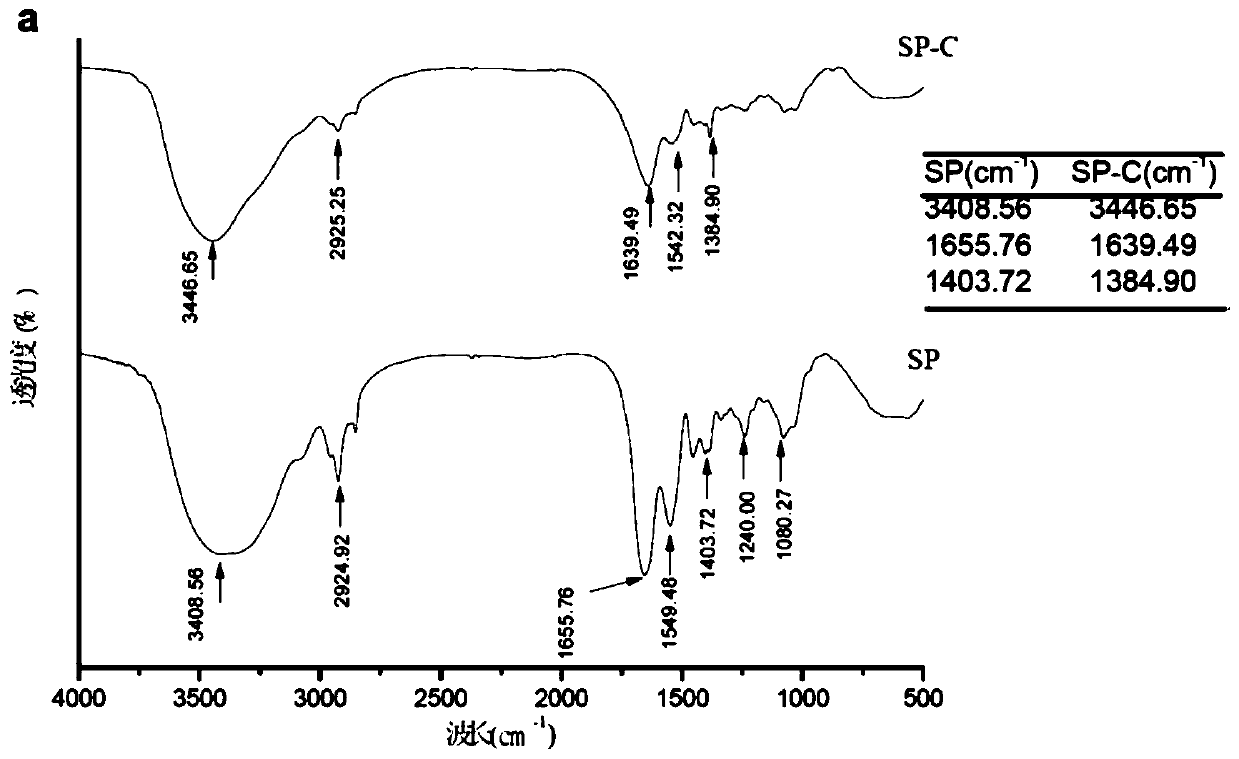
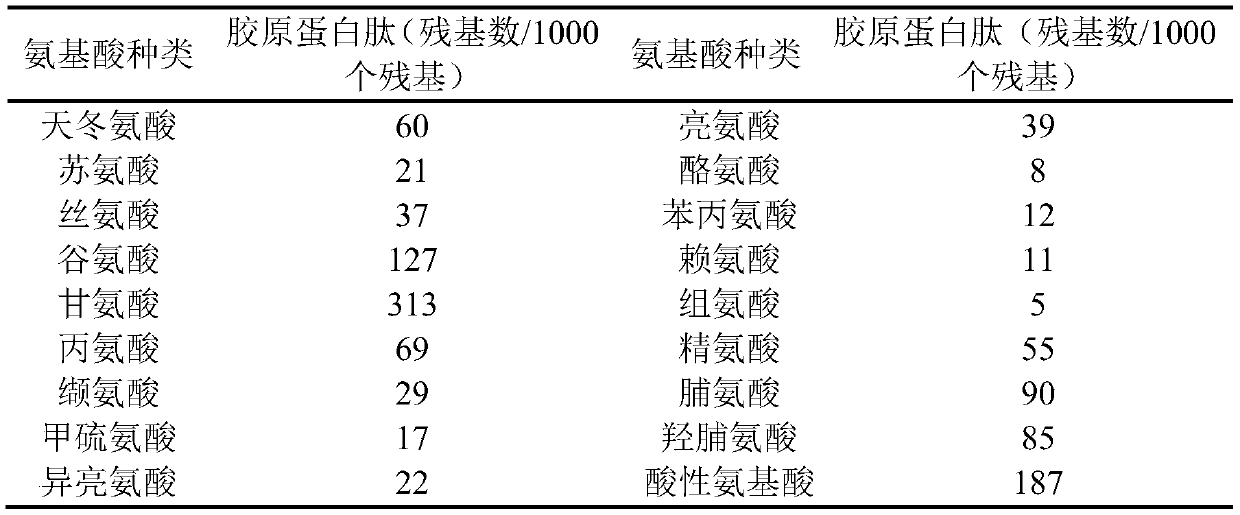
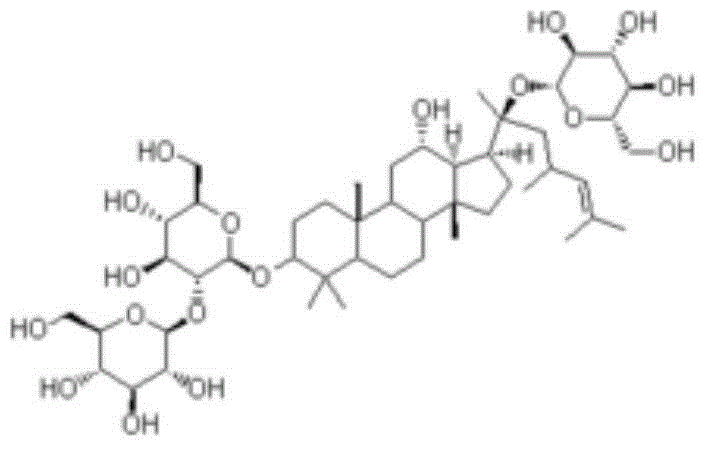
Patents
Literature
37 results about "Mad Cow Diseases" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Large-scale preparation method for foot-and-mouth disease totivirus marked vaccine with high yield, high purity and high safety and product thereof
ActiveCN104491855AMark stableEnsure safetyMicroorganism based processesAntiviralsSucroseUltrafiltration
The invention discloses a large-scale preparation method for foot-and-mouth disease totivirus marked vaccine with high yield, high purity and high safety and a product thereof. The method comprises the following steps: a)collecting a virus solution; b)performing deep filtration on a membrane, performing ultrafiltration and performing enzymolysis on nuclease; c)purifying through a strong anion exchange adsorption bed or an adsorption film; d)depositing by PEG, extracting by chloroform-isoamyl aleohl; e)inactivating; F)performing density gradient centrifugation on an inactivation liquid through cane sugar and purifying; g)performing ultrafiltration dialysis and aseptic filtration; and h)reserving a stock solution or emulsifying. The provided foot-and-mouth disease totivirus marked vaccine antigen is uniform and complete foot-and-mouth virus particle, The vaccine is injected into body, so animal infection and immunization can be completely distinguished, does not contain foot-and-mouth disease virus non-structural protein and other virus particle, and does not contain animal-based foreign protein, polypeptide and oligopeptides, animal latent anaphylactic reaction, carcinogenesis and latent risk such as mad cow disease for causing animal infectious diseases due to vaccine injection can be effectively reduced, and the vaccine has no influence on animal food safety and trade.
Owner:吕宏亮 +2
Method for identifying source constituent in meat product by micro-satellite labeling technique
InactiveCN101435001APrevent incomingEnsure safetyMicrobiological testing/measurementBiotechnologyAdditive ingredient
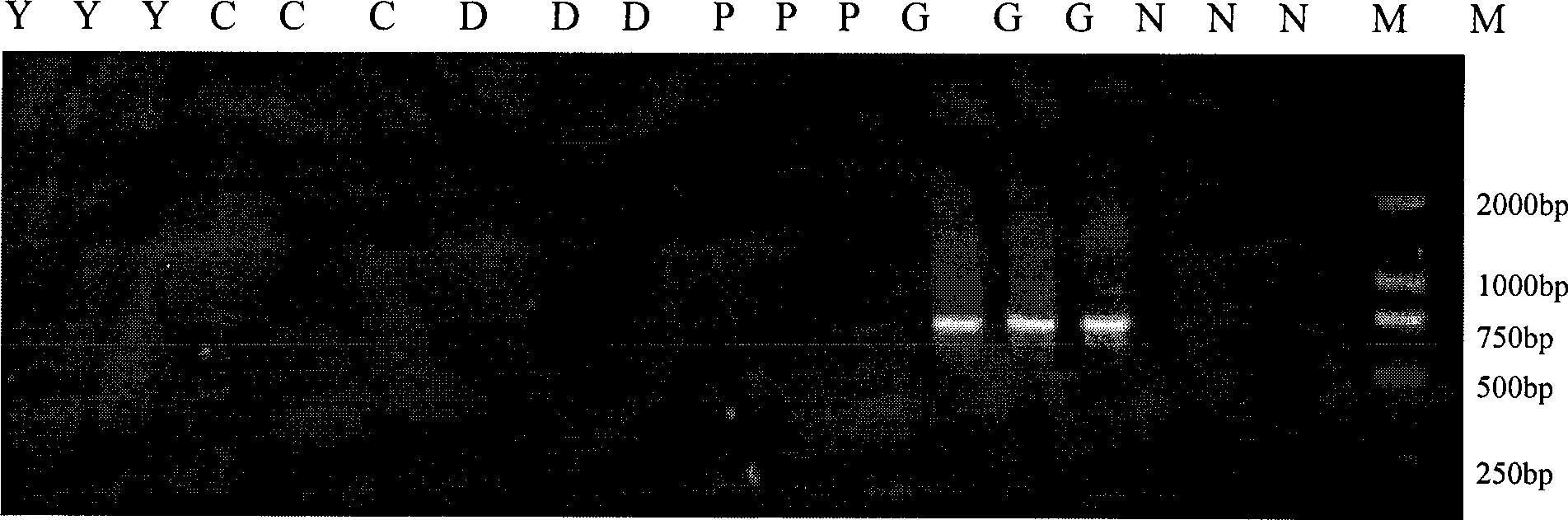
The invention relates to a method which uses microsatellite marking technology for identifying the animal origin ingredients in meat products, belonging to the biological high-tech field. The use of the microsatellite marking technology for identifying the origin ingredients of cattle, sheep, pigs, dogs, chickens and ducks in the meat products comprises the extraction of genomic DNA in meat products, the conducting of PCR reaction, the PCR product detection and primer screening, and the identification of samples. The method selects microsatellite marks with specific property among the types and universal property in the type, designs specific primers, establishes PCR detection methods, and identifies the origin ingredients of cattle, sheep, pigs, dogs, chickens and ducks in the meat products according to the size and the availability of amplification fragment. The invention has the advantages of rapidity, simplicity, accuracy, strong specificity and high sensitiveness. The invention is suitable for being promoted and used for detecting multitudinous samples. The invention also has great significance for preventing the mad cow disease from being spread into China and the illegal businessmen from concealing the commodity elements, and ensuring national security and the health of people.
Owner:NANJING AGRICULTURAL UNIVERSITY
Piezoelectric bio-chip for detecting pathogen of mad cow disease and thereon preparation
InactiveUS20060121531A1High-precision detectionEasy to operateBioreactor/fermenter combinationsBiological substance pretreatmentsQuarantineOrganism
The present invention relates to the device for animal inspection and quarantine and the preparation method thereof. The present invention is especially applicable to the detection of the pathogen of bovine spongiform encephalopathy (BSE) (also known as “mad cow disease”). The present invention employs a piezoelectric chip, a microelectrode array and a common electrode fixed on the lower side surface and upper side surface of the piezoelectric chip, respectively, and a BSE PrP antibody array to constitute the piezoelectric biochip for the detection of the BSE pathogen. The BSE PrP antibodies are immobilized on the electrodes of the microelectrode array in a format corresponding uniquely to the electrodes of the microelectrode array by adsorbing, bonding, cross-linking, embedding or self-assembly process. The combination of the piezoelectric biochip and a detector constitutes the piezoelectric biochip detection system for the BSE pathogen. When the antibodies react with the corresponding PrPs immunochemically, the information about the PrPs can be detected at real time by measuring the resonant frequency, and the PrPs thus can be analyzed qualitatively and quantitatively. The present invention is applicable to the early, effective and rapid detection of the BSE pathogen.
Owner:PEOPLES REPUBLIC OF CHINA BEIJING ENTRY EXIT INSPECTION & QUARANTINE BUREAU +1
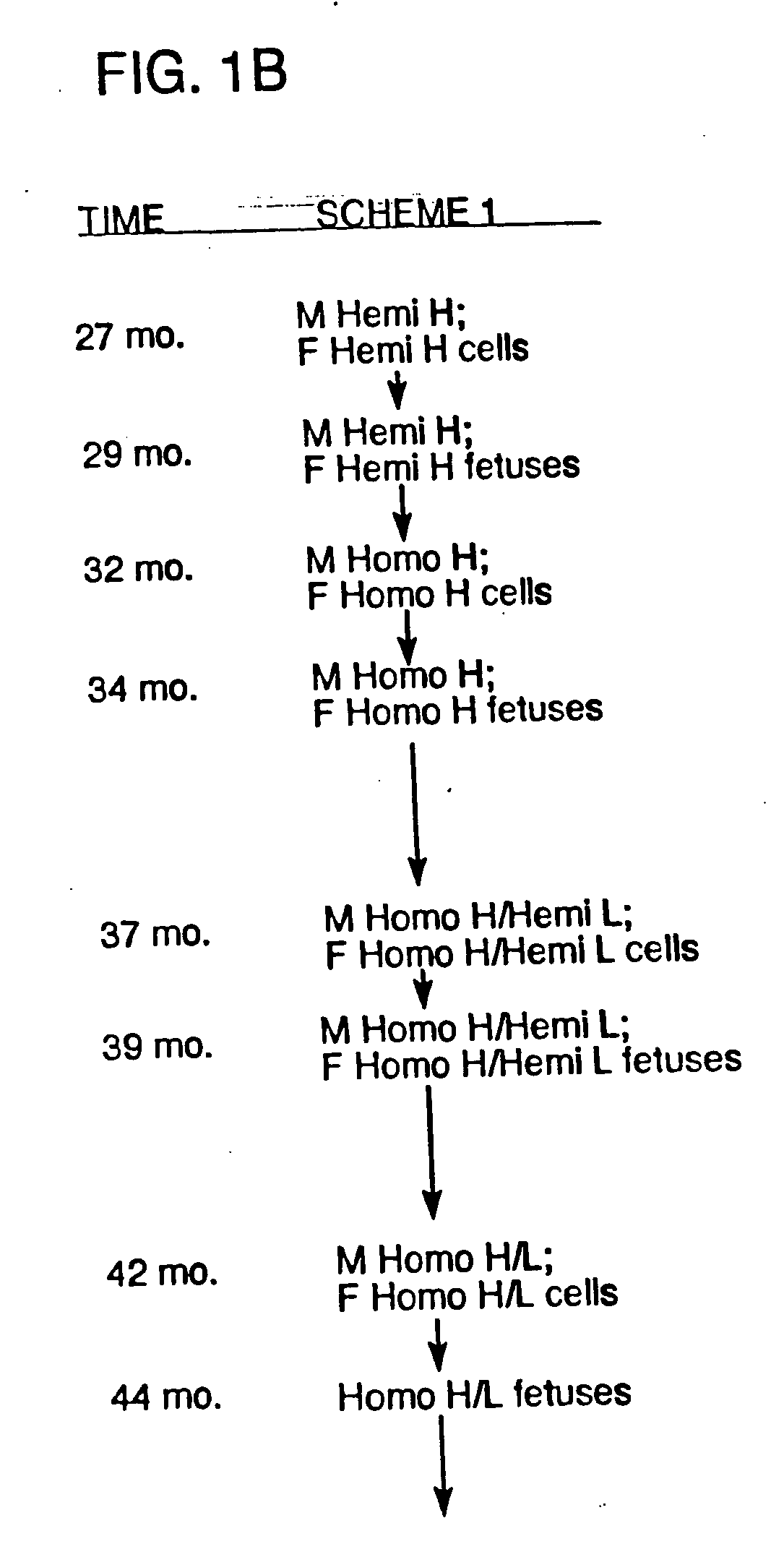
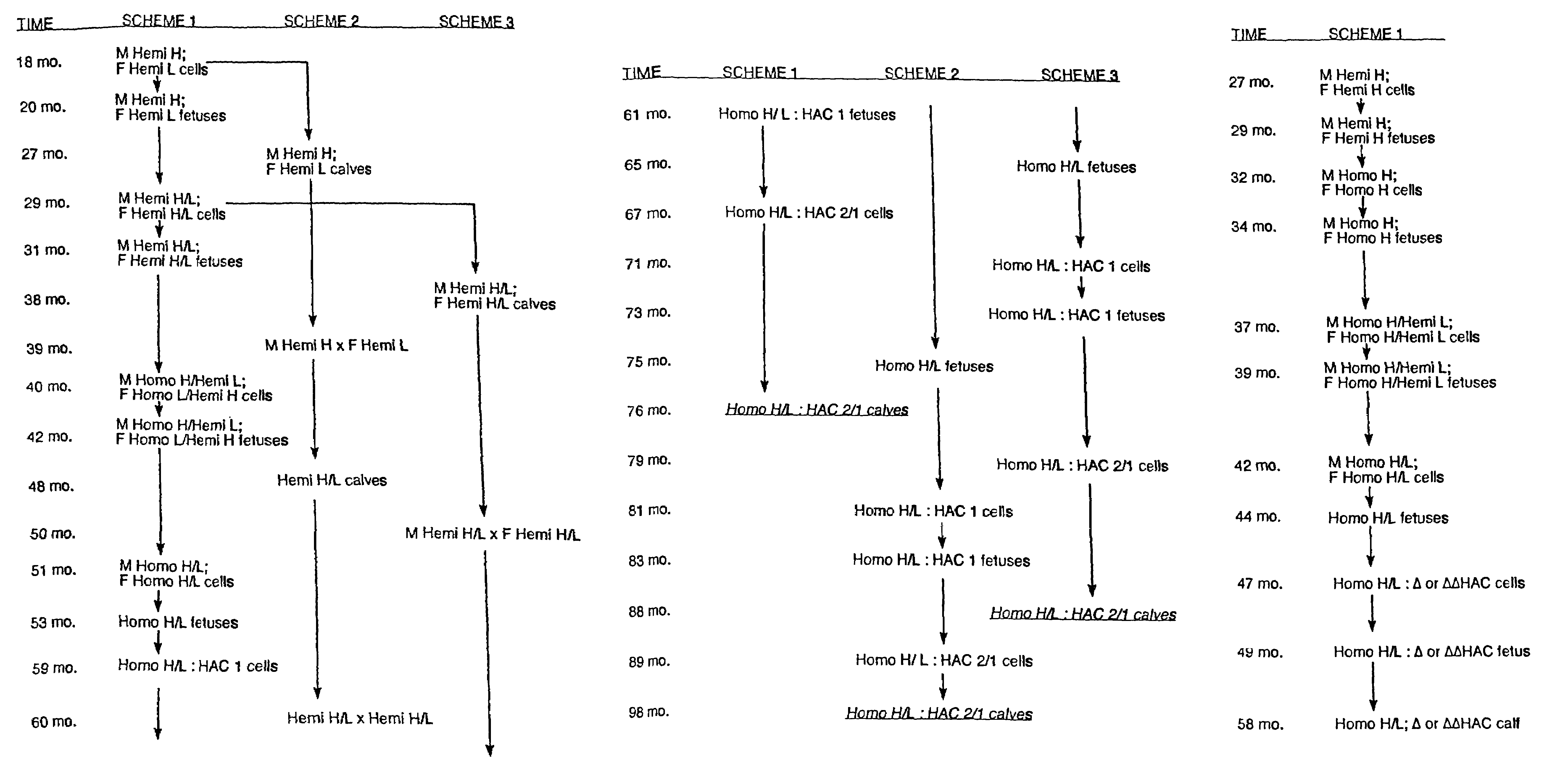
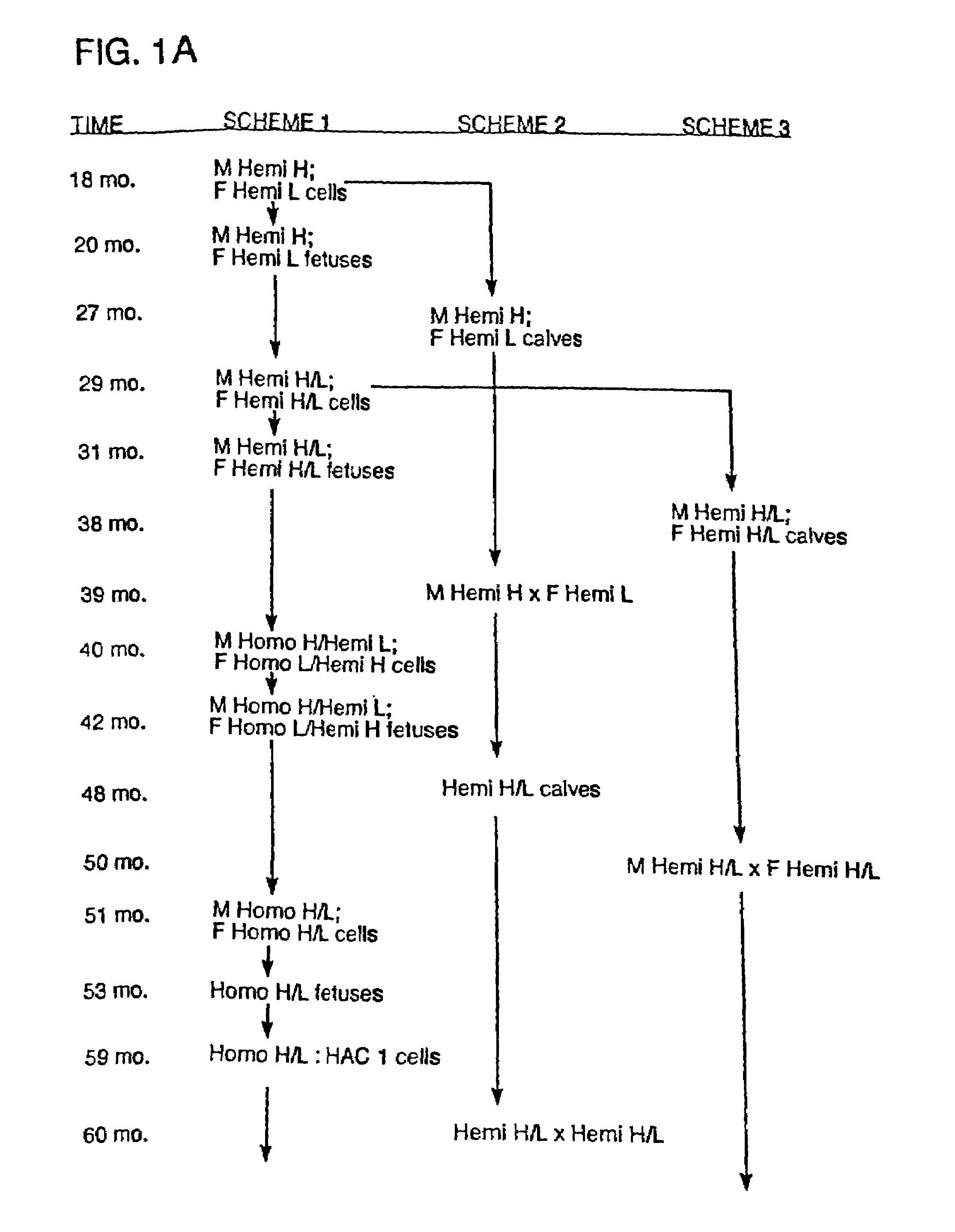
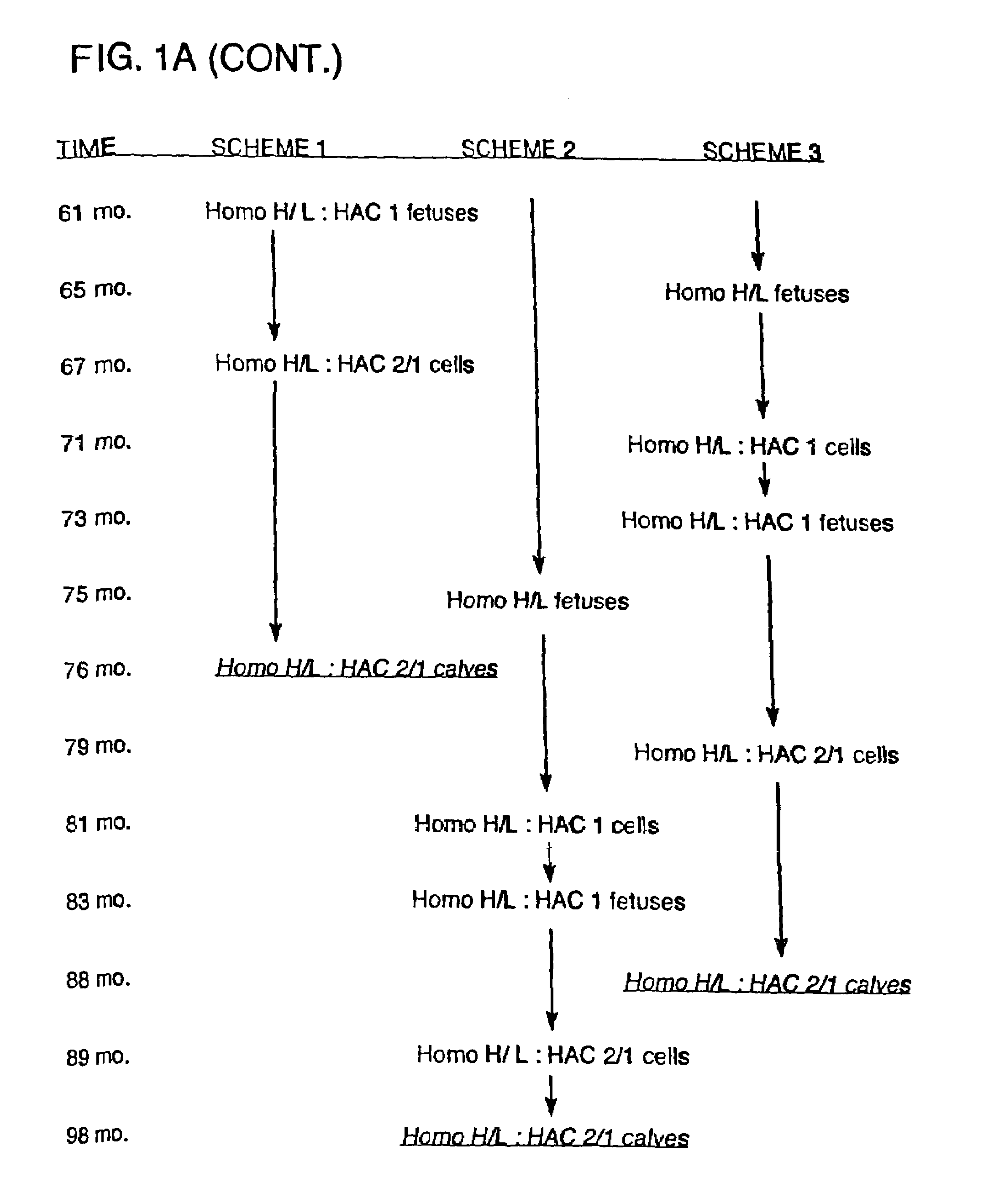
Transgenic ungulates having reduced prion protein activity and uses thereof
ActiveUS20050097627A1Animal cellsMicrobiological testing/measurementBiotechnologyGenetically engineered
The invention provides cloned transgenic ungulates (e.g., bovines) in which prion protein activity is reduced by one or more genetically engineered mutations. Desirably, these transgenic bovines are also genetically modified to express xenogenous (e.g., human) antibodies. Because of their resistance to prion-related diseases such as bovine spongiform encephalopy (also known as mad cow disease), these bovines are a safer source of human antibodies for pharmaceutical uses and a safer source of agricultural products.
Owner:SAB LLC
Method and apparatus for infrared sterilization
InactiveUS6863864B1Less energyAvoid the needMaterial analysis using wave/particle radiationElectric discharge tubesEscherichia coliEating Utensils
The disclosed invention relates to sterilization by infrared radiation to eliminate pathogenic bacteria such as Salmonella, E. Coli 0157:H7 and E. Coli (EXEC) from articles such as medical, dental and veterinary instruments, as well as from tableware and eating utensils. The invention further relates to sterilization of soil, military and agriculture equipment to eliminate pathogenic bacteria such as hepatitis, AIDS, and anthrax, and prions such as mad cow disease using infrared radiation. Sterilization is performed by exposing the article to infrared radiation generated from heating elements positioned in an enclosed chamber.
Owner:U S STERILIZER CORP
Modulation of prion expression
ActiveUS20110269818A1Lower Level RequirementsDownsized mannerOrganic active ingredientsNervous disorderHuntingtons choreaScrapie Virus
Disclosed herein are compounds and methods for decreasing PrP and preventing, ameliorating, or treating a prion disease or conformational neurodegenerative disorder, in an individual in need thereof. Examples of disease conditions that can be ameliorated with the administration of antisense compounds targeted to PrP include Creutzfeldt-Jakob disease (CJD); variant Creutzfeldt-Jakob Disease (vCJD); Gerstmann-Straussler-Scheinker syndrome; fatal familial insomnia; kuru; Bovine Spongiform Encephalopathy (BSE), e.g. “mad cow disease”; Chronic Wasting Disease (CWD); scrapie; transmissible mink encephalopathy; feline spongiform encephalopathy; ungulate spongiform encephalopathy; Alzheimer's disease; Parkinson's disease; Huntington's disease; and Amyotrophic Lateral Sclerosis (ALS).
Owner:IONIS PHARMA INC
Assay for species sources
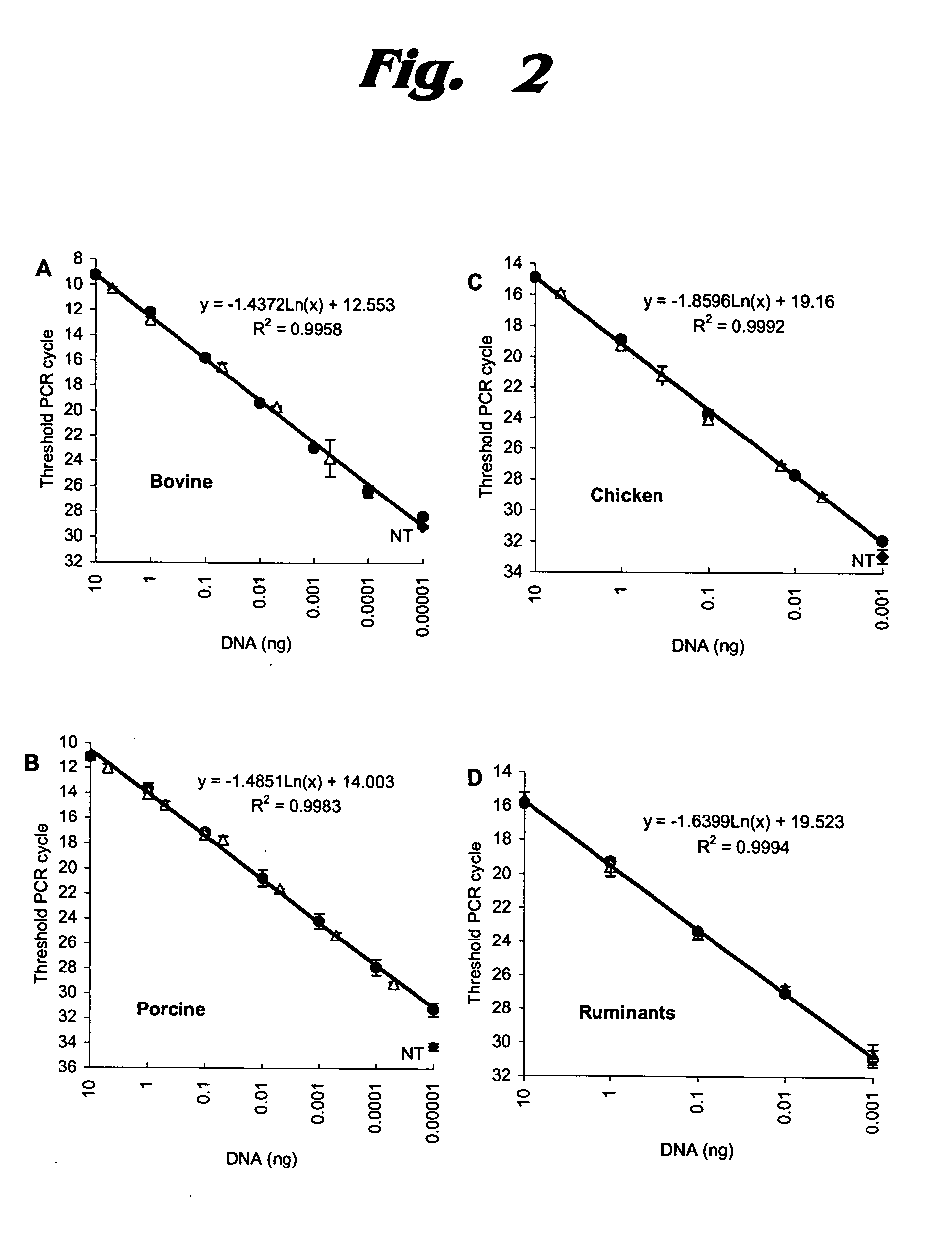
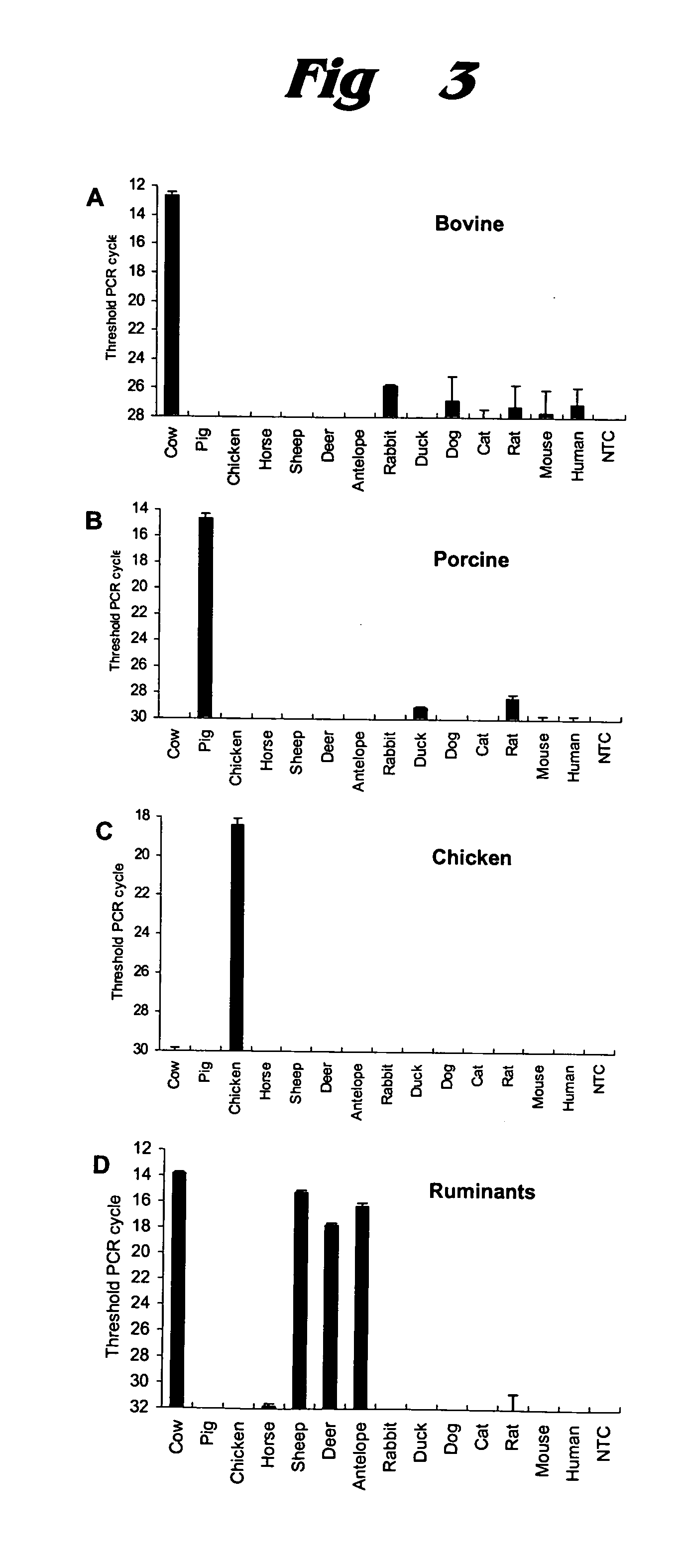
ActiveUS20050112592A1Low costAvoid the needSugar derivativesMicrobiological testing/measurementBiotechnologyMad Cow Diseases
A family of PCR assays is disclosed for determining, both qualitatively and quantitatively, presence of material from a predetermined species source and for quantifying the amount of such material. The assays are based respectively on SINEs uniquely characteristic of pig species, cow species, chicken species, and ruminant sub-order, and having a high copy number. The assays disclosed permit rapid, inexpensive evaluation of meat samples to facilitate elimination from their diet of pork or beef by persons desiring to avoid such food sources; as well as the assay of cattle feed to determine presence therein of ruminant-source proteins, which are a potential source of bovine spongiform encephalopathy (BSE), commonly referred to as “mad cow disease.” The assays amplify the predetermined unique SINEs and the resulting amplified mixture is then evaluated qualitatively by electrophoresis on gel containing ethidium bromide or quantitatively by SYBR Green-based detection or TaqMan chemistry. The invention also extends to kits, primers, and other products used in connection with the assays. The amplicons are selected to be from about 100 to 170 bp long.
Owner:RELIAGENE TECH +2
Analogs of choline for neuroprotection and cognitive enhancement in neurodegenerative disorders
InactiveUS6881738B2Prevent further deteriorationIncrease awarenessBiocideNervous disorderParaneoplastic cerebellar degenerationDementia with Lewy bodies
The present invention relates to novel analogs of choline and methods of use or treatment of neurodegenerative disorders and / or conditions such as Parkinson's disease, Huntington disease, Alzheimer's disease and related disorders such as amyotrophic lateral sclerosis, spinal muscular atrophy, Friedrich's ataxia, Pick's disease, Bassen-Kornzweig syndrome, Refsom's disease, retinal degeneration, Cruetzfelt-Jacob syndrome or prion disease (mad cow disease), dementia with Lewy bodies, schizophrenia, paraneoplastic cerebellar degeneration and neurodegenerative conditions caused by stroke. The present compounds are effective to treat any neurological condition where acetylcholine transmission neurons and their target cells are affected. Compounds according to the present invention are effective to alleviate and / or reverse the effects of a neurodegenerative condition, prevent further deterioration and / or enhance cognition and memory in patients suffering from neurodegenerative disorders, especially Alzheimer's disease.
Owner:AUGUSTA UNIV RES INST INC +1
Fetal cattle blood serum production technology
InactiveCN101112334AIncrease costChange the situation of relying on foreign importsDispersed particle separationMammal material medical ingredientsObstetricsFiltration
The present invention relates to a manufacturing technique of fetal bovine serum. The present invention includes the full closed aseptic collection, low temperature separation and microporous membrane filtration, which is characterized in that: the present invention carries out the caesarean section of a cow which is approximately eight months pregnant, lateral decubitus restraint method is adopted before the delivery, and the anesthesia adopts the method with the combination of general anesthesia and local anesthesia at the surgical site. The full closed aseptic cardiac puncture for blood sampling is carried out on the fetal bovine; after the collection of the blood, a large-capacity refrigerated centrifuge is used for centrifugation of 30 minutes as the rotation speed of 3400 turns per minute, and the separated serum is collected in a sterile container under the local class 100 area for rapid cryopreservation. The present invention has the advantage that: the price of the imported fetal bovine serum is higher, which reaches about 6,000 RMB per liter. The imported fetal bovine serum from the United States and Europe not only has high cost, but also has the hidden dangers of mad cow disease. The price of the fetal bovine serum which is produced by our manufacturing technique is just 4000 to 5000 RMB per liter, which completely changes the status that the fetal bovine serum in China entirely dependents on the foreign imports.
Owner:兰州民科生物技术中心
Modulation of prion expression
ActiveUS8669102B2Organic active ingredientsNervous disorderHuntingtons choreaFatal familial insomnia
Owner:IONIS PHARMA INC
Transgenic ungulates having reduced prion protein activity and uses thereof
The invention provides cloned transgenic ungulates (e.g., bovines) in which prion protein activity is reduced by one or more genetically engineered mutations. Desirably, these transgenic bovines are also genetically modified to express xenogenous (e.g., human) antibodies. Because of their resistance to prion-related diseases such as bovine spongiform encephalopy (also known as mad cow disease), these bovines are a safer source of human antibodies for pharmaceutical uses and safer source of agricultural products.
Owner:KYOWA HAKKO KIRIN CO LTD
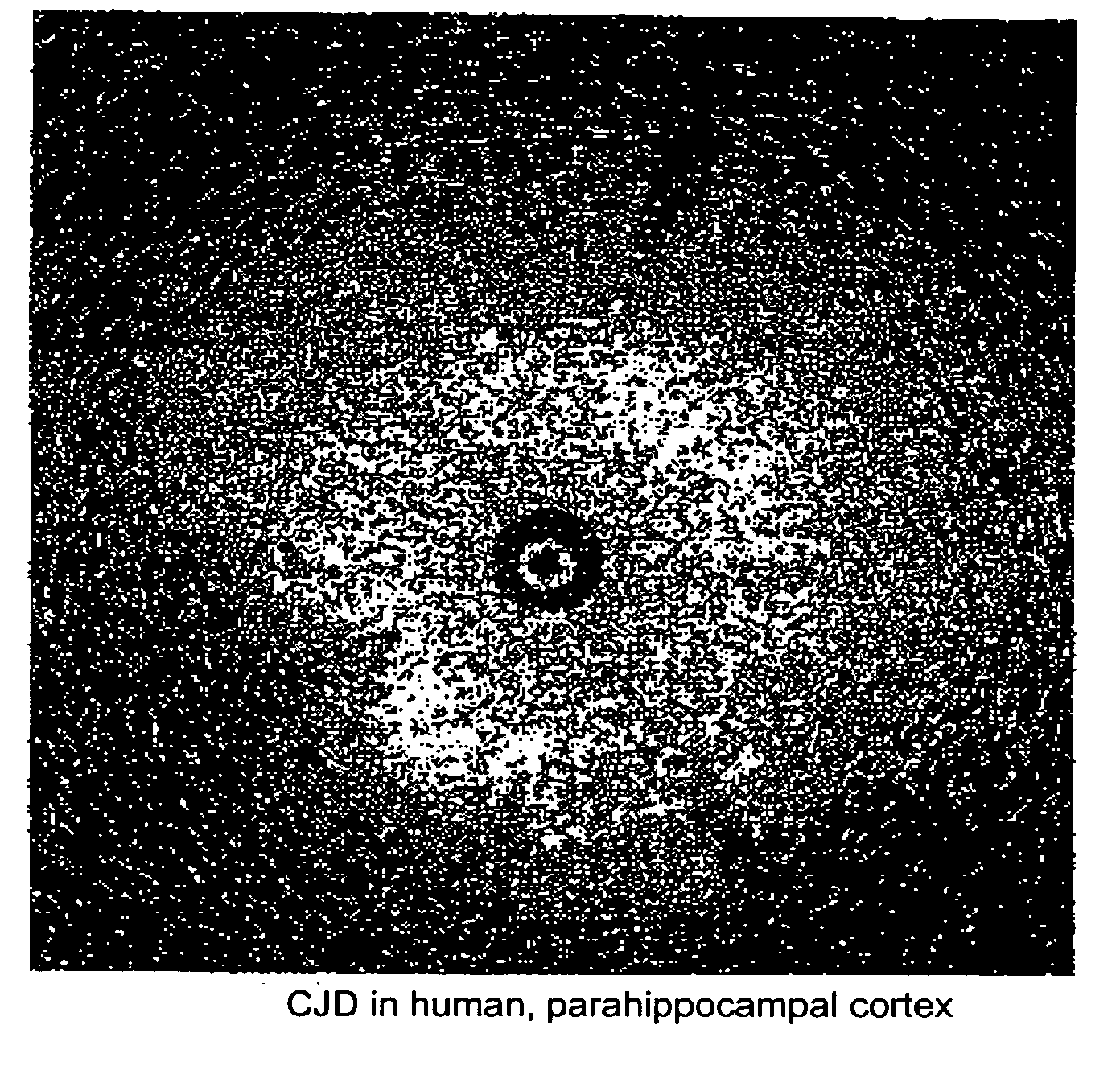
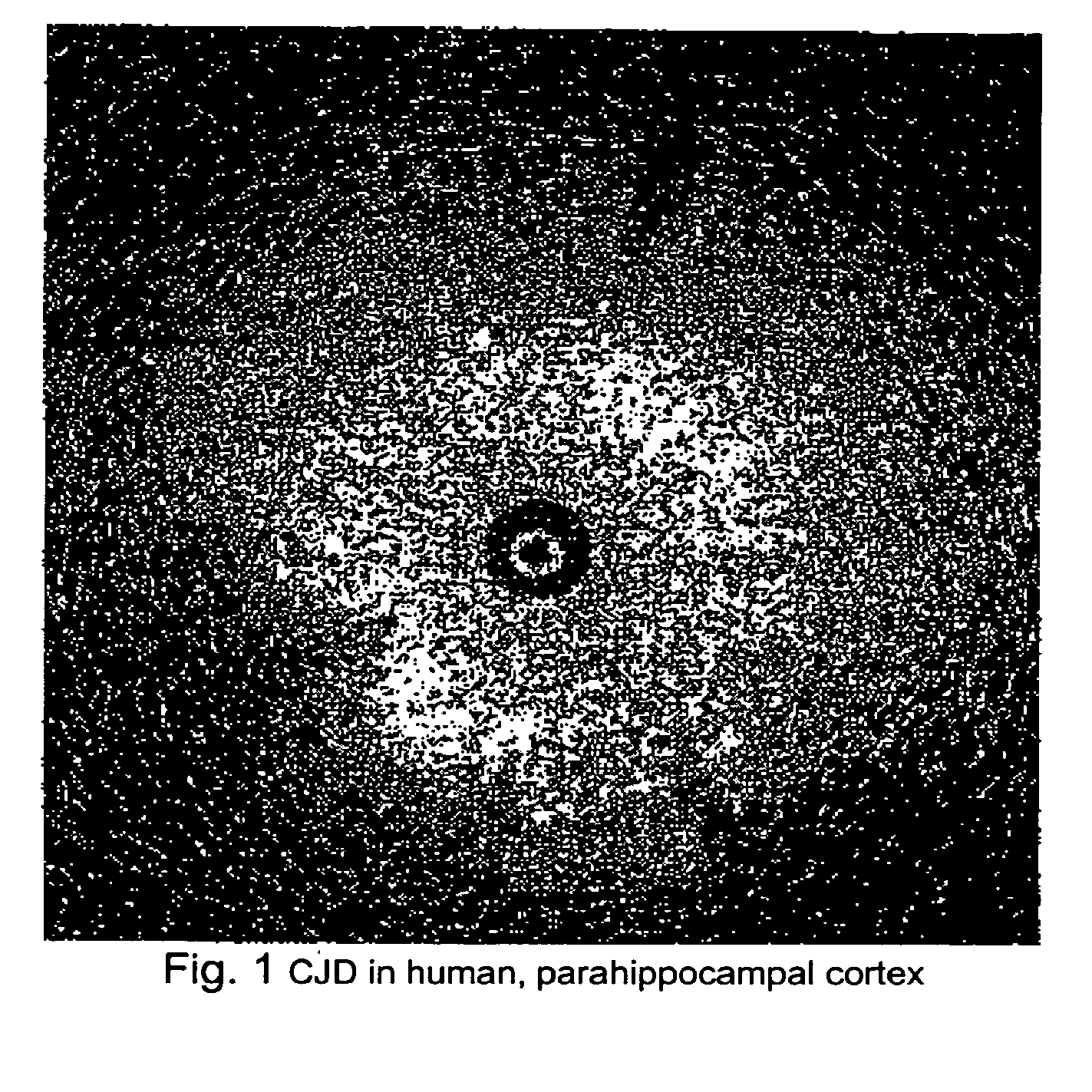
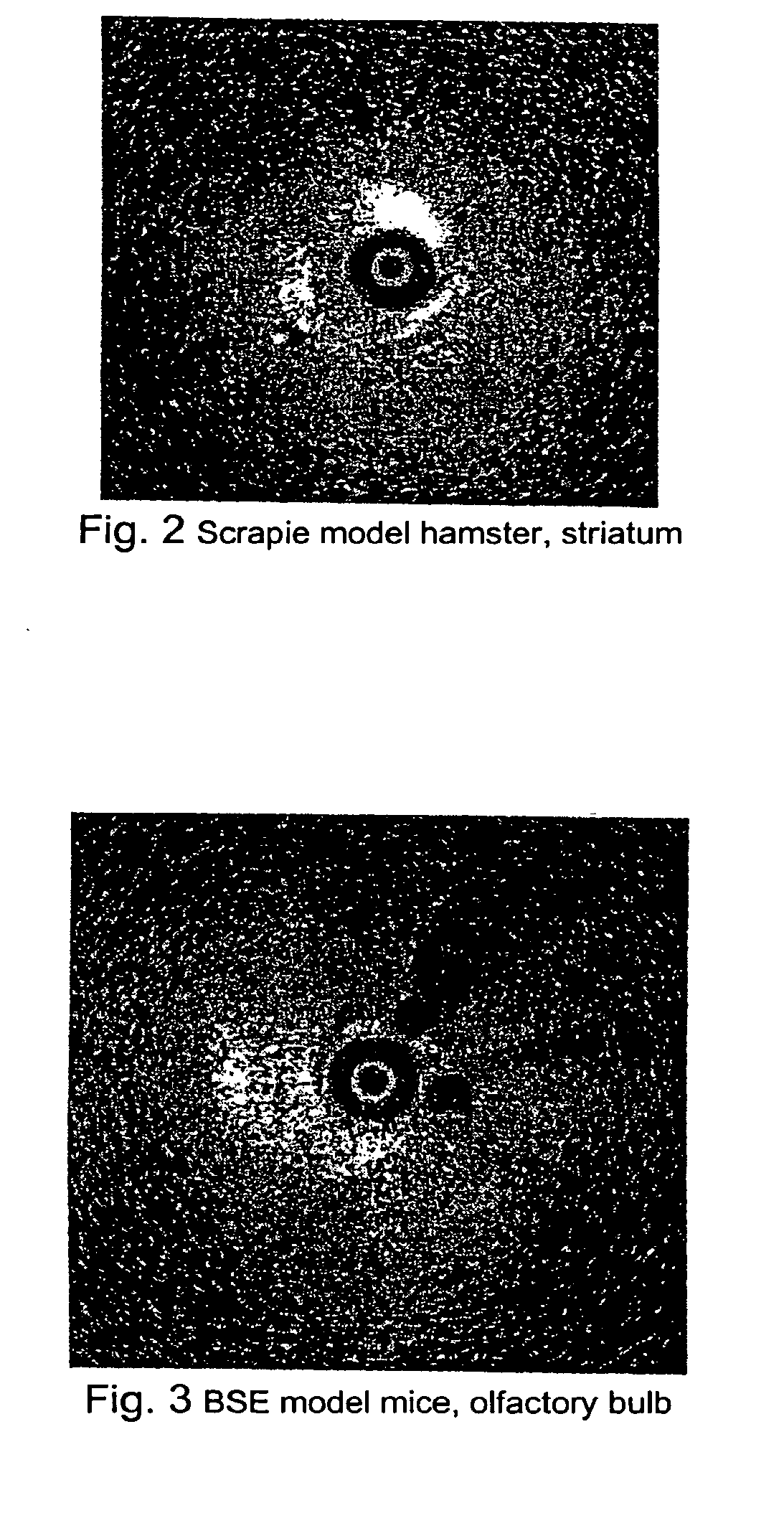
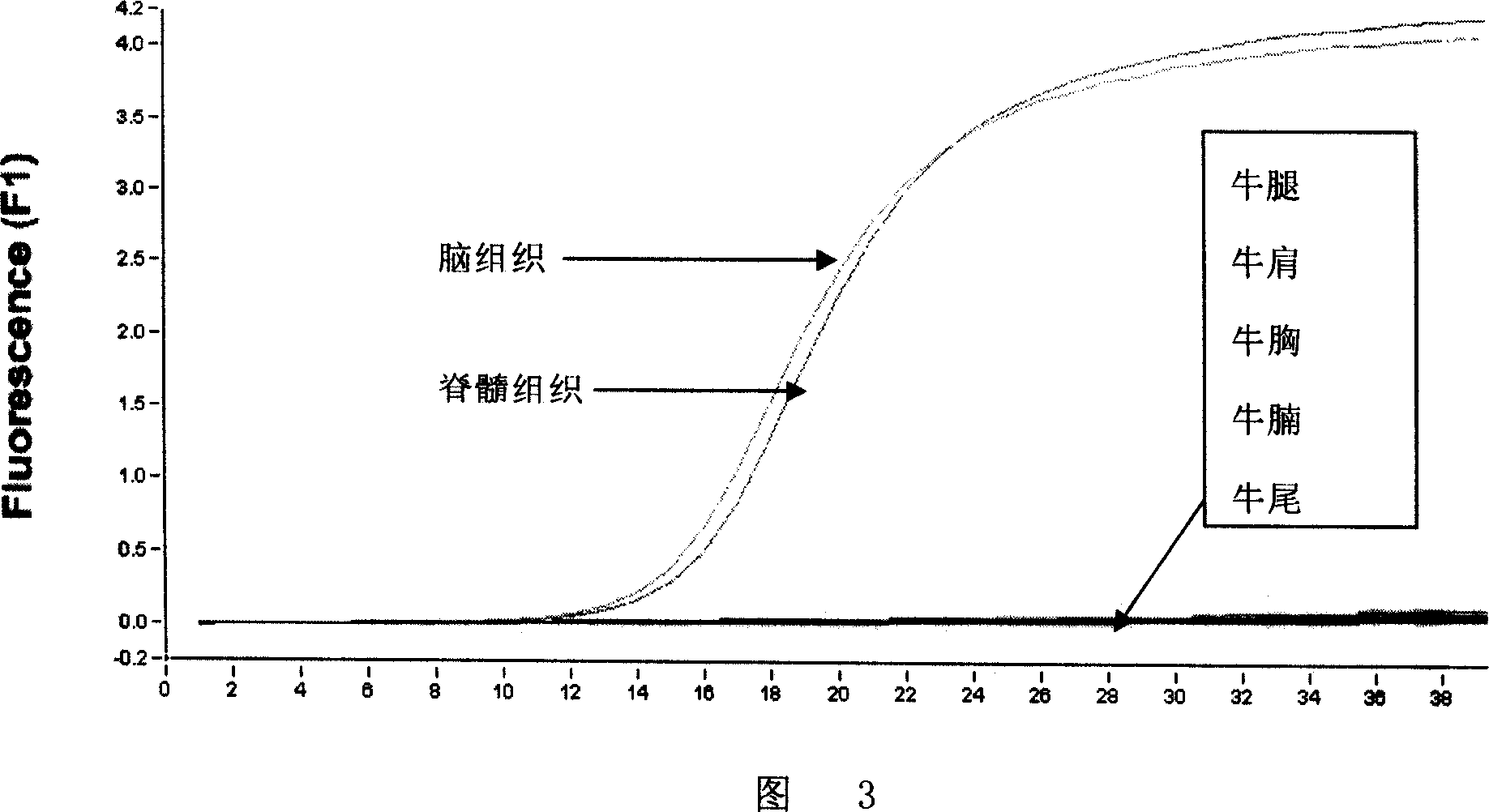
Methods for rapid screening of mad cow disease and other transmissible spongiform encephalopathies
Methods for diagnosing altered neuropathology in an animal are disclosed, wherein said methods comprise imaging brain, spinal cord, or other neural tissue of the animal, analyzing the appearance of the tissue, and determining whether the appearance of the tissue is altered relative to corresponding unaltered tissue. Also disclosed are methods for diagnosing spongiform encephalopathies in an animal, wherein said methods comprise imaging brain, spinal cord, or other neural tissues of the animal, analyzing the appearance of vacuoles in the tissue, and determining whether the appearance of the vacuoles in the tissue is altered relative to corresponding spongiform encephalopathy-free tissue. Also disclosed are automated methods for diagnosing altered neuropathy and spongiform encephalopathies.
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS +1
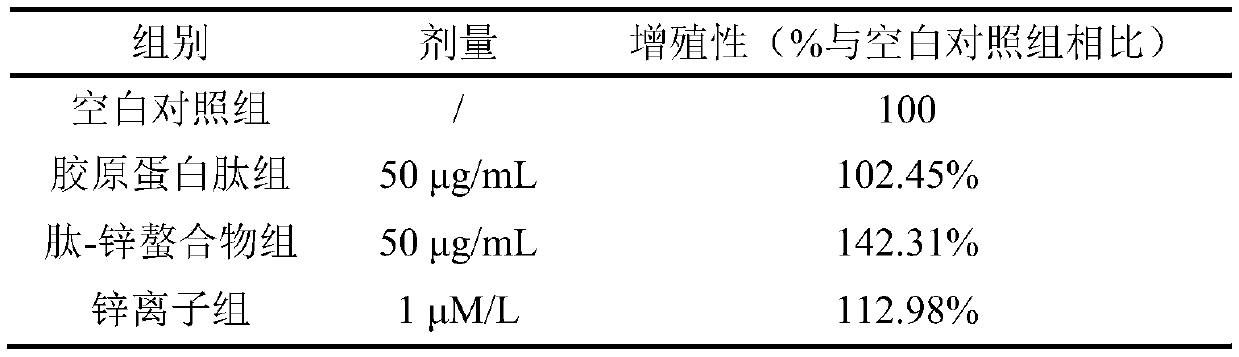
Preparation method of collagen peptide-zinc chelate
InactiveCN110590938AAvoid mad cow diseaseReduce riskConnective tissue peptidesPeptide preparation methodsSide effectTerrestrial animal
The invention discloses a preparation method of a collagen peptide-zinc chelate. According to the preparation method, fresh shellfish is used as raw materials; modified shellfish pallium water-solublecollagen is obtained by a hot extraction method; then, the water-soluble collagen is hydrolyzed by protease to obtain collagen peptide; the collagen peptide is chelated with metallic zinc ions; and the collagen peptide-zinc chelate is obtained. The method provided by the invention has the advantages that processing wastes of marine organisms are used as the raw materials, the acquisition path issimple and convenient; the cost is low; the chelation rate is high; and risks such as mad cow diseases and foot-and-mouth diseases possibly occurring in terrestrial animal collagen are avoided. Meanwhile, compared with zinc ions, the peptide-zinc chelate obtained after the collagen peptide is chelated with the metallic zinc ions has the advantages that the toxic and side effects are small; the synergistic effect of the collagen peptide and the zinc ions can be achieved; and safe and good-effect anti-osteoporosis functional food can be provided for customers.
Owner:HAINAN UNIVERSITY
Prion-free transgenic ungulates
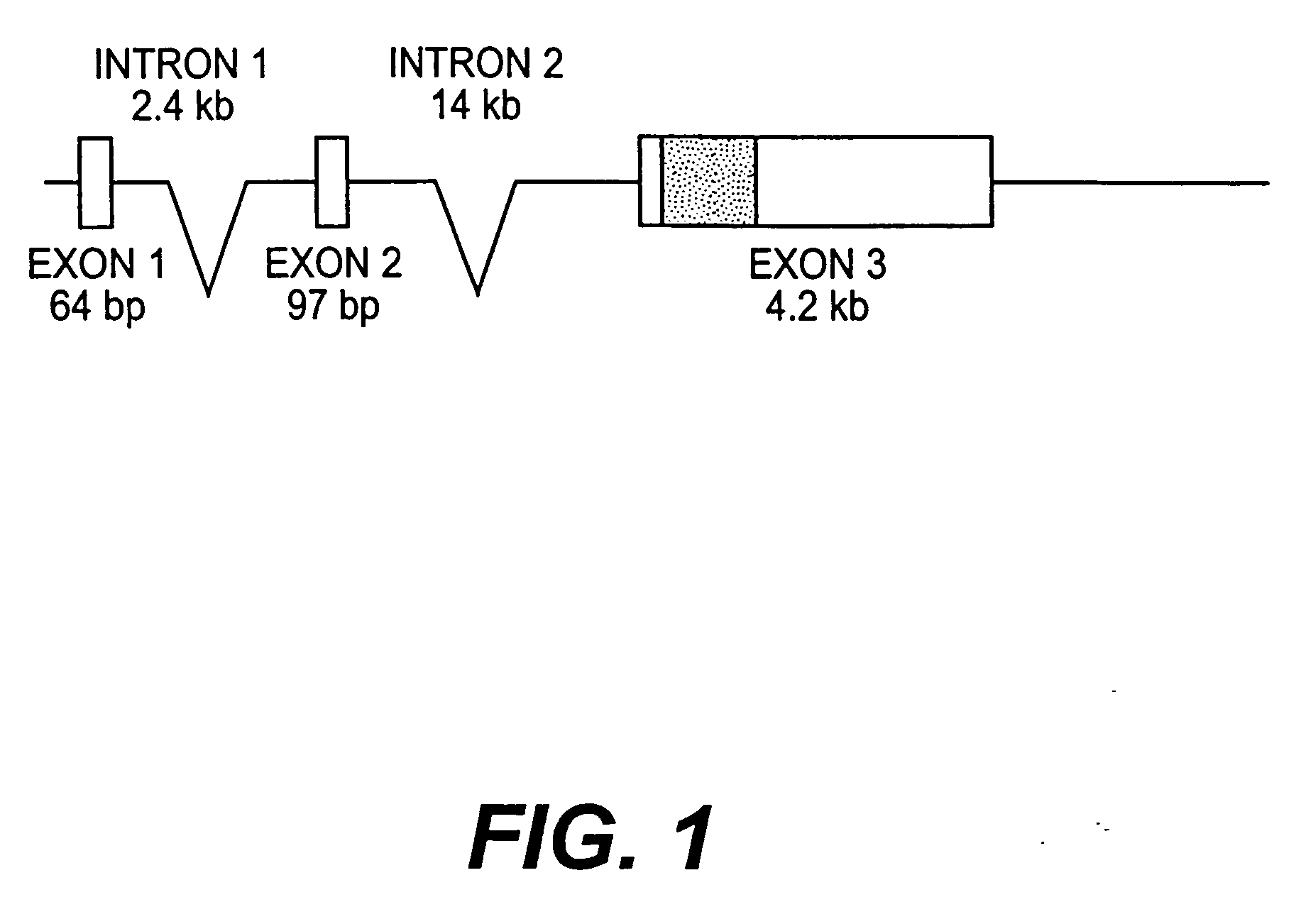
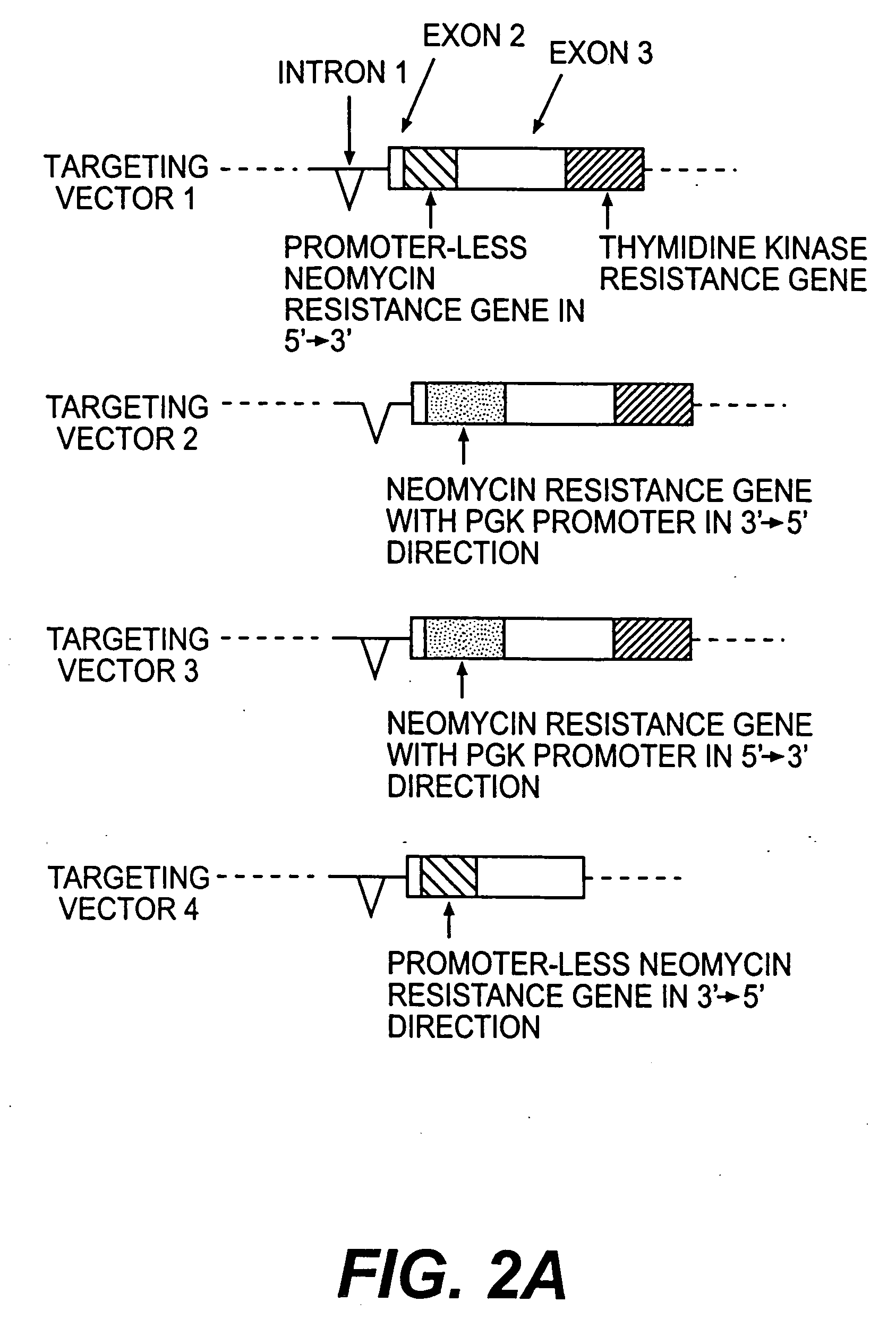
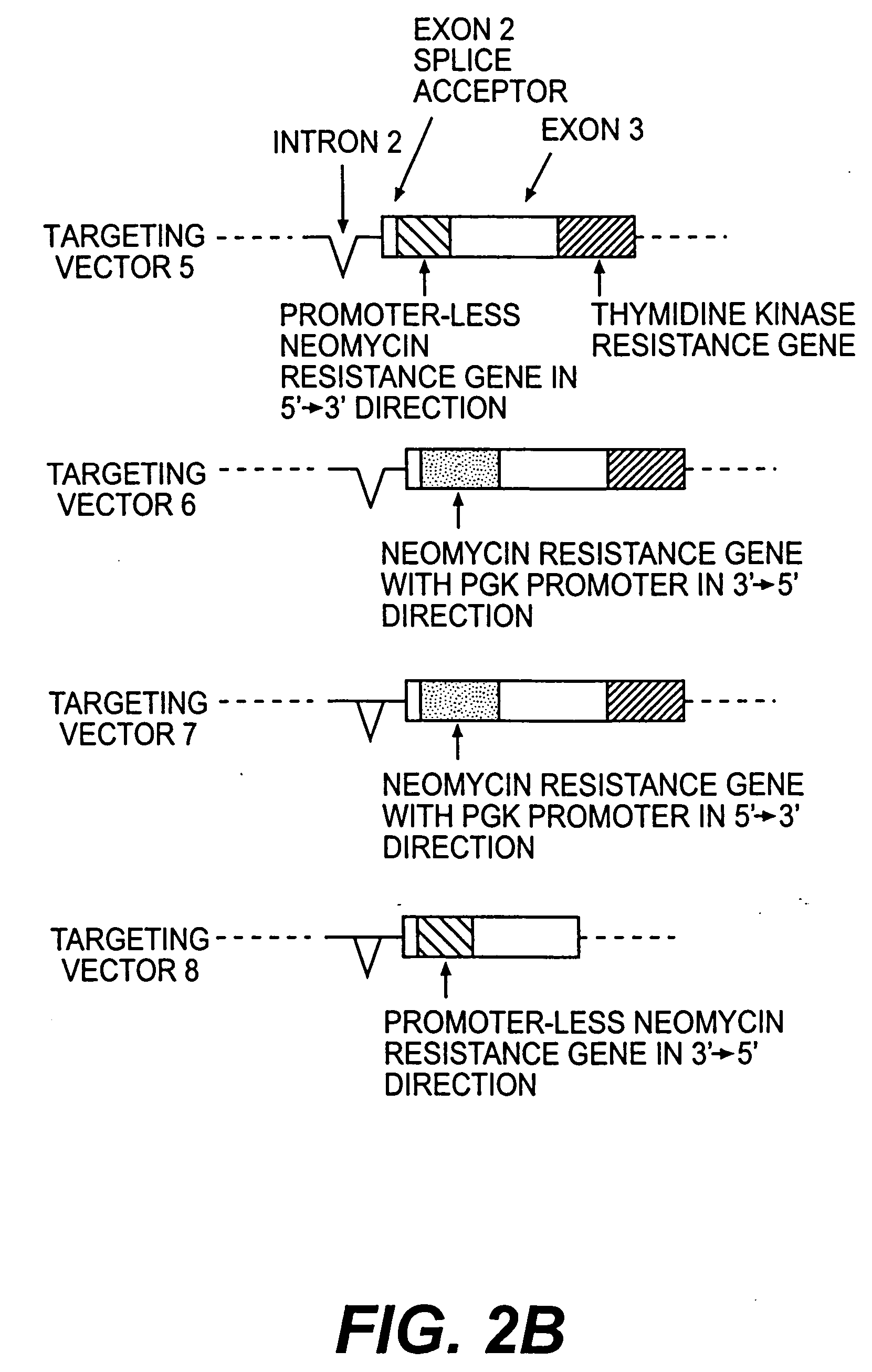
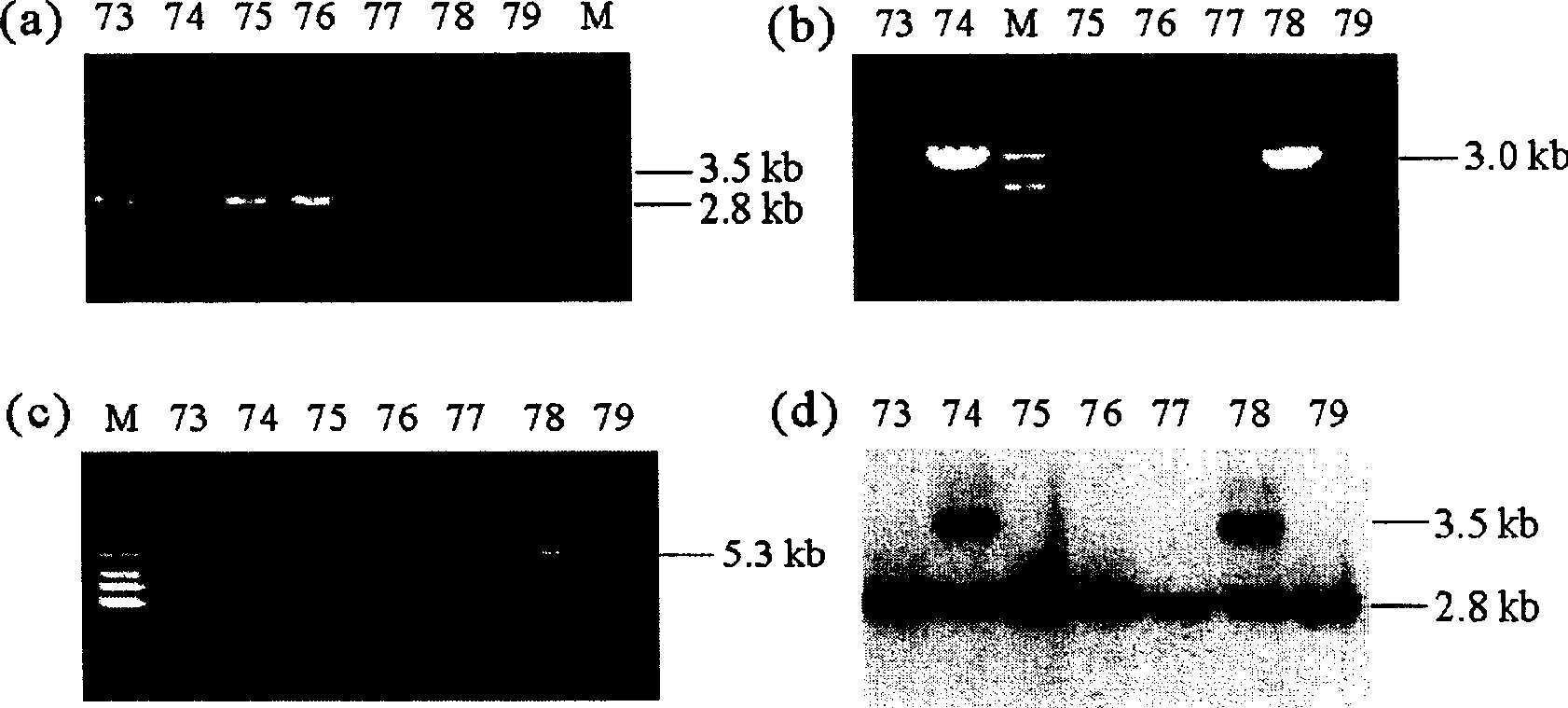
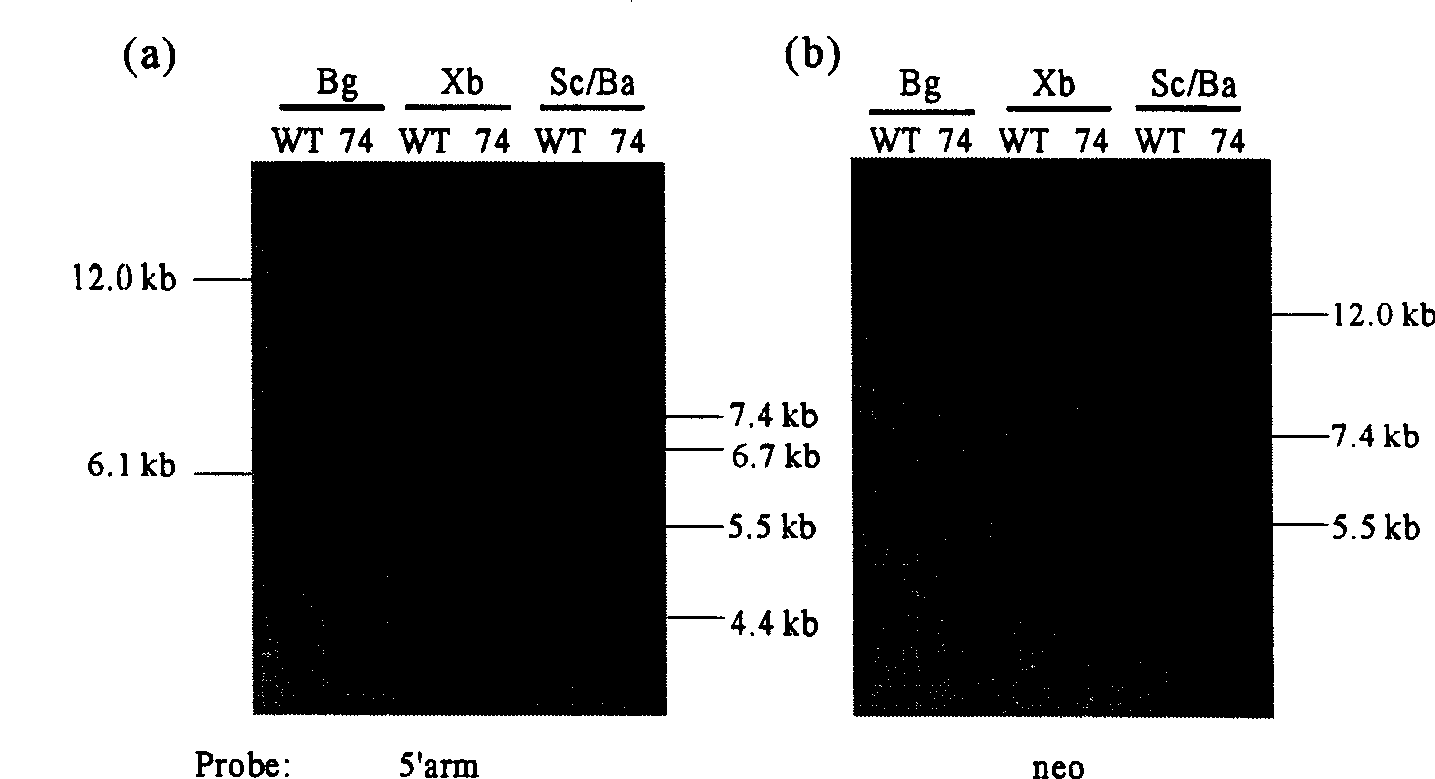
InactiveUS20050216963A1Less susceptibility and susceptibilityFermentationAnimals/human peptidesPrion geneHeifer calf
Transgenic and cloned ungulates and particularly cloned cattle are disclosed, wherein such cattle contain a deletion or disruption of the prion gene locus and do not express functional prion protein, and are not susceptible to prion-related diseases such as bovine spongiform encephalopy or Mad Cow Disease.
Owner:UNIV OF MASSACHUSETTS
Novel application of ginsenoside Rd in preparing drug for preventing and/or treating microglial cell mediated diseases
InactiveCN104958307ASignificant preventionGood treatment effectOrganic active ingredientsNervous disorderMS multiple sclerosisAIDS dementia
The invention relates to a novel application of ginsenoside Rd in preparing a drug for treating microglial cell mediated diseases, and particularly relates to a novel application of ginsenoside Rd in preparing a drug for treating diseases such as disseminated sclerosis, amyotrophic lateral sclerosis, HIV-associated dementia, mad cow disease and the like.
Owner:GUANGXI WUZHOU PHARMA GRP
Culture medium for preparing tetanus toxin and application thereof
The invention discloses a culture medium for preparing tetanus toxin and a method for preparing the tetanus toxin from the culture medium. The culture medium contains an organic nitrogen source of a yeast source, the mass percent of the organic nitrogen source of an animal source and / or a plant source in the organic nitrogen source of the culture medium is smaller than 1-50%. According to the culture medium, the risk of introducing zoonotic diseases such as mad cow disease is avoided, strain anabiosis and seed culture can be met, and produced toxin is also far higher than the requirements of the existing standard. The operation of preparing the tetanus toxin by utilizing the culture medium is simple, the cost is low, and the method is favorable for industrial production.
Owner:CANSINO BIOLOGICS INC
Meal replacement powder rich in fish albumen glue and preparation method thereof
PendingCN112425783AHigh in proteinMeets requirementsProtein composition from fishFood dryingBiotechnologyPolygonum fagopyrum
The invention discloses meal replacement powder rich in fish albumen glue. The meal replacement powder is prepared from the following components: fish albumen glue, buckwheat flour, cane sugar, blacksesame seed powder, black bean powder, red date powder, red bean powder, apple powder, hawthorn fruit powder and deionized water. The prepared meal replacement powder is high in calcium ion content, amino acid content and protein content and various in product taste, contains the composite taste of fruits and cereals, the mixed taste of nuts and the like, and meets the requirements of young people, middle-aged people and old people for healthy products at present. Meanwhile, the problems of food safety caused by infectious diseases such as mad cow disease and foot-and-mouth disease, dietary habits of religious persons and special requirements of vegetarians are solved, and the application range of the fish albumen glue in the field of food is expanded.
Owner:JIANGXI NORMAL UNIV
Fishskin collagen aggregates based bone conductive material and preparation method and application thereof
ActiveCN108888806AEliminates separation and extraction stepsNatural bionic three-dimensional structure is goodTissue regenerationMicrocapsulesFiberConductive materials
The invention belongs to the field of biological materials and particularly relates to a fishskin collagen aggregates based bone conductive material and a preparation method and application thereof. The material can be widely applied to the field of bone tissue repair and plastic surgery, and the bone conductive material is obtained by taking natural fishskin collagen aggregates with moderate treatment as carriers and plant polyphenols and aldehyde groups as media and solidifying fish bones subjected to pelletizing processing on the surface and internal pores of the fishskin collagen aggregates. The material can be directly prepared by taking the fishskin collagen aggregates as the carriers, better natural bionic three-dimensional structure and better mechanical properties are achieved, and potential risk of human and zoonotic diseases such as mad cow disease and foot and mouth diseased caused by the use of animal source materials such as pigs and cows is avoided.
Owner:HUAZHONG AGRI UNIV
Nicergoline lyophilized preparation with excellent stability
The invention provides a nicergoline lyophilized preparation with excellent stability. The nicergoline lyophilized preparation is prepared by freeze-drying a nicergoline drug solution, and each 1000 gof the nicergoline drug solution comprises: 2-4 g of nicergoline, 0.5-1 g of tartaric acid, 0.2-9 g of sodium chloride, 20-40 g of an excipient, and an appropriate amount of water for injection, andthe pH value of the drug solution is 3.0-5.0. In the formula of the lyophilized preparation, mannitol is used to replacing lactose in an original development agent to be a lyophilized excipient, which can avoid the risks of allergies, pyrogens, and mad cow disease caused by lactose as an injection adjuvant, and overcomes the instability caused by the absence of lactose in the prior art. The mostnotable feature of the preparation is the combination of mannitol, sorbitol and the like with sodium chloride, and the nicergoline for injection still has excellent stability without using of nicergoline. The lyophilized product of the invention has rapid reconstitution and loose appearance, and is convenient for transportation and preservation and clinical use.
Owner:湖北科莱维生物药业有限公司
A kind of nicergoline freeze-dried preparation with excellent stability
The invention provides a kind of nicergoline preparation with excellent stability, which is prepared by freeze-drying a nicergoline medicinal solution, and every 1000 g of the nicergoline medicinal solution contains: 2-4 g of nicergoline, 0.5 g of tartaric acid -1g, 0.2-9g of sodium chloride, 20-40g of excipients, and an appropriate amount of water for injection, and the pH of the drug solution is 3.0-5.0. In the formula of the present invention, the lactose in the original preparation is replaced by mannitol as the freeze-drying excipient, which can avoid the risk of allergy, pyrogen, and mad cow disease caused by lactose used as an injection auxiliary material from the source, And it overcomes the instability caused by not using lactose in the prior art. The most notable feature of the present invention is that mannitol, sorbitol, etc. are combined with sodium chloride, so that Nicergoline for injection can still have excellent stability without using lactose. The freeze-dried product of the invention is reconstituted quickly, has a loose appearance, and is convenient for transportation, preservation and clinical use.
Owner:湖北科莱维生物药业有限公司
Prion-free transgenic ungulates
InactiveUS20100024047A1Less susceptibility and susceptibilitySugar derivativesMicroinjection basedPrion geneGenetics
Owner:GOOD DEBORAH J +1
Process for preparing prion protein gene-free domestic animals
InactiveCN1970749BSignificant application valueUnderstanding Normal Physiological FunctionClimate change adaptationVector-based foreign material introductionScrapieSomatic cell
The present invention discloses a preparation method for prion protein gene knockout domestic animals. The process is characterized by: knocking off the prion protein gene of domestic animals on a cellular level; and preparing prion protein gene knockout domestic animals through somatic cell cloning method. The function of the prion protein of domestic animals is inactivated. The functional prion protein is not expressed, thus the domestic animals can resist to relative disease of prion protein, such as scrapie, bovine spongiform encephalopathy (mad cow disease) and the like.
Owner:SHANGHAI GENON BIOENG
Work method of mad cow disease detection and timely anesthesia flight microrobot
InactiveCN106781355ADiscovered in timeOvercome monitoringRespiratorsTransmission systemsPhysical medicine and rehabilitationRemote control
The invention discloses a work method of a mad cow disease detection and timely anesthesia flight microrobot. The method mainly comprises the steps that a remote control signal is received through a wireless remote control unit, and flight is performed according to the remote control signal; physiological information parameters are collected by aiming at specific cultivation cattle; the collected physiological information parameters and the prestored mad cow disease parameter indexes are compared one by one; if the indexes are not exceeded, the physiological information parameters are continuously collected; if the parameter value of the mad cow disease is exceeded, anesthesia injection is performed on the detected cattle. Therefore, the defect that the fully automatic monitoring on the cattle herds and the timely control on the cattle with the mad cow diseases cannot be realized in the prior art can be overcome; the goal of fully automatically monitoring the state information of the cattle herds is realized; the mad cattle and the sick cattle can be timely found; the measures can be taken in time.
Owner:HECHI UNIV
Method for rapid screening of mad cow disease and other transmissible spongiform encephalopathies
InactiveUS20090209867A1Microbiological testing/measurementDisease diagnosisSpinal cordTransmissible spongiform encephalopathy
Owner:TANG CHA MIN +1
Health-care mixed feed for cattle and sheep
InactiveCN109170249AMeeting nutritional needsIncrease growth rateFood processingAnimal feeding stuffBiotechnologyMalaria
The present invention relates to the technical field of feeds and particularly relates to a health-care mixed feed for cattle and sheep. The health-care mixed feed comprises the following raw materials in parts by weight: 100 parts of corn, 40-80 parts of straw powder, 20-70 parts of protein-enriched mulberry leaves, 5-20 parts of bruguiera gymnorrhiza leaves, 5-10 parts of compound vitamins special for cattle and sheep, 3-8 parts of an additive and 0-3 parts of wax gourd leaves. The bruguiera gymnorrhiza leaves are rich in sugars, lipids and proteins, and high in nutritional value, have certain malaria-preventing effects, and avoid malaria in the cattle and sheep. In summer, an appropriate amount of the wax gourd leaves can be added to play functions of treating malaria and diarrhea, canalso eliminate heat and prevent diseases, and have very good health-care functions. The high-protein protein-enriched mulberry leaves and bruguiera gymnorrhiza leaves are used to replace animal meat or bone meal and can effectively prevent spread of hereditary diseases of mad cow disease, etc.
Owner:GANSU AONONG FEED TECH CO LTD
Nucleotide sequence, method and agent case for detecting bovine spongiform encephalitis specific risk substance in beef
InactiveCN101033488AImprove stabilityDoes not affect detection sensitivityMicrobiological testing/measurementDNA/RNA fragmentationAgricultural scienceNucleotide sequencing
The invention discloses a kind of nucleotide sequence for detecting the special risk of mad cow disease of beef and its method as well as reagent. The group of nucleotide sequence has a base sequence shown in the sequence from SEQ ID No.1 to SEQ ID No.3. The advantage of the invention is that: it judges whether there is GFAP mRNA in the beef or not to determine whether the beef is contaminated by the risk of mad cow disease. Comparing with the traditional ELISA technology, the method is more sensitive, rapid, and better stability.
Owner:BEIJING ENTRY EXIT INSPECTION & QUARANTINE BUREAU INSPECTION & QUARANTINE TECH CENT
Method for preparing fish scale gelatin plasma substitute injection
InactiveCN105457012ALow costHigh biosecurityHydrolysed protein ingredientsPharmaceutical delivery mechanismCross-linkSide effect
The invention relates to a method for preparing a fish scale gelatin plasma substitute injection. The method for preparing the fish scale gelatin plasma substitute injection includes the steps that fish scale gelatin serves as a raw material, and the plasma substitute injection is obtained through the production processes of gelatin degradation, cross-linking modification, electrolyte adding, adsorption, filtering, filling, sterilization, lamp inspection, packaging and the like. The method has the advantages that the plasma substitute injection is prepared from the fish scale gelatin, the low cost of the raw material is guaranteed, potential safety risks, caused when a gelatin raw material from animals such as pigs and cows possibly carries epidemic diseases such as a foot-and-mouth disease and a mad cow disease, of the human body are avoided, and a fish scale gelatin plasma substitute has higher biosecurity; succinic anhydride is used as a cross-linking agent, and therefore the toxic and side effects on the human body are avoided; a proper quantity of electrolytes are added, and therefore it is guaranteed that electrolyte disturbance can not be caused when a large quantity of fish scale gelatin plasma substitutes are injected; as a porous titanium bar and a microporous filtering film are combined to carry out classified filtering, the filtering speed is greatly increased, and the method is better suitable for large-scale production.
Owner:WUHAN HAIJIYA BIOTECH CO LTD
Transgenic ungulates having reduced prion protein activity and uses thereof
InactiveCN100526460CEasy to produceFused cellsGenetic engineeringAntiendomysial antibodiesTransgenesis
The present invention provides cloned transgenic ungulates (eg, cattle) in which the activity of a prion protein is reduced by one or more genetically engineered mutations. Desirably, these transgenic cattle are also genetically modified to express exogenous (eg, human) antibodies. These cattle are a safer source of human antibodies for medicinal purposes and a safer source of agricultural products due to their protection against prion-related diseases such as bovine spongiform encephalopathy (also known as mad cow disease).
Owner:KYOWA HAKKO KIRIN CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com