Patents
Literature
45 results about "Spongiform encephalopathy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method and kit for extracting prion protein
InactiveUS6150172AMethod is fastThe testing process is simplePeptide preparation methodsDepsipeptidesIonic strengthSheep brain
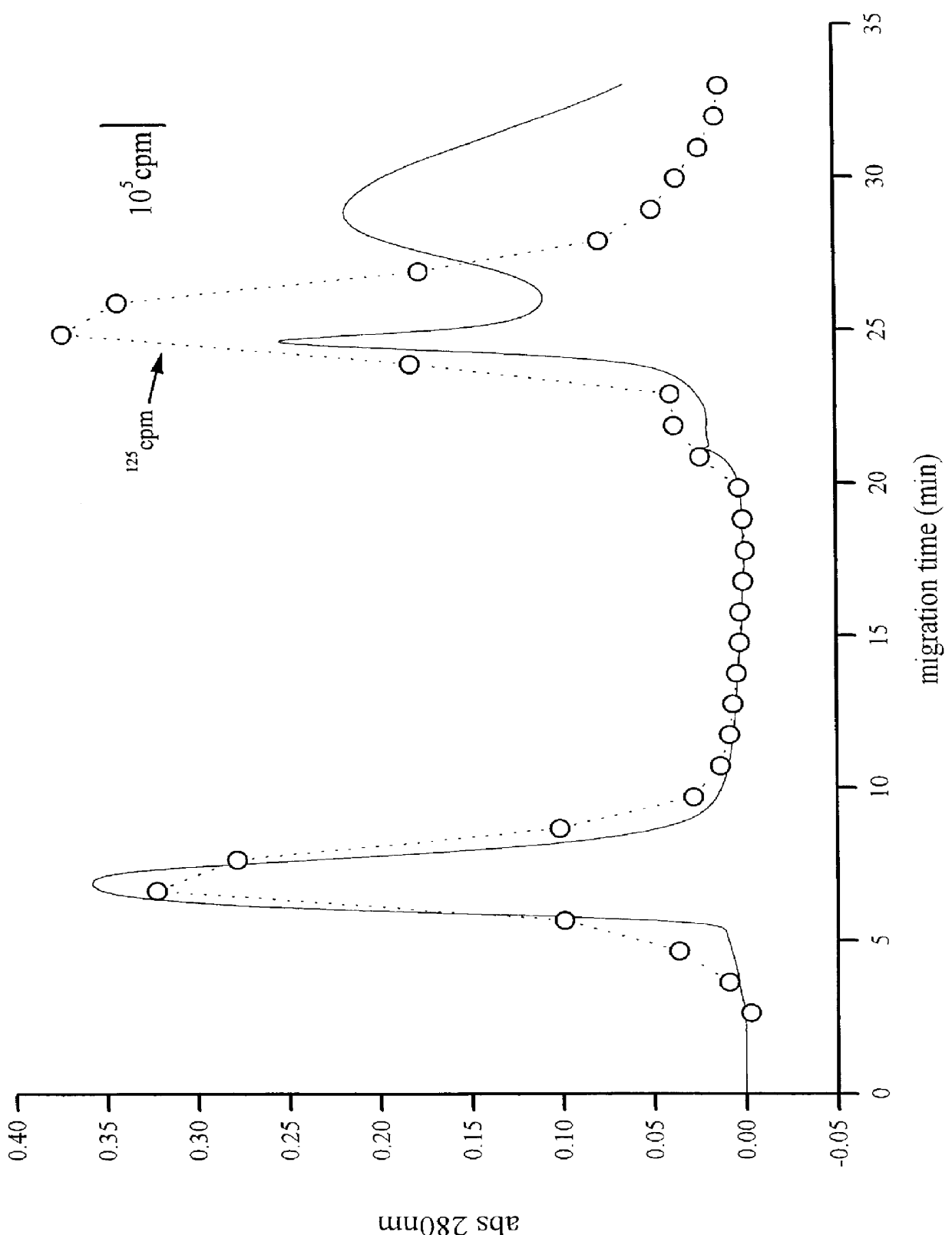
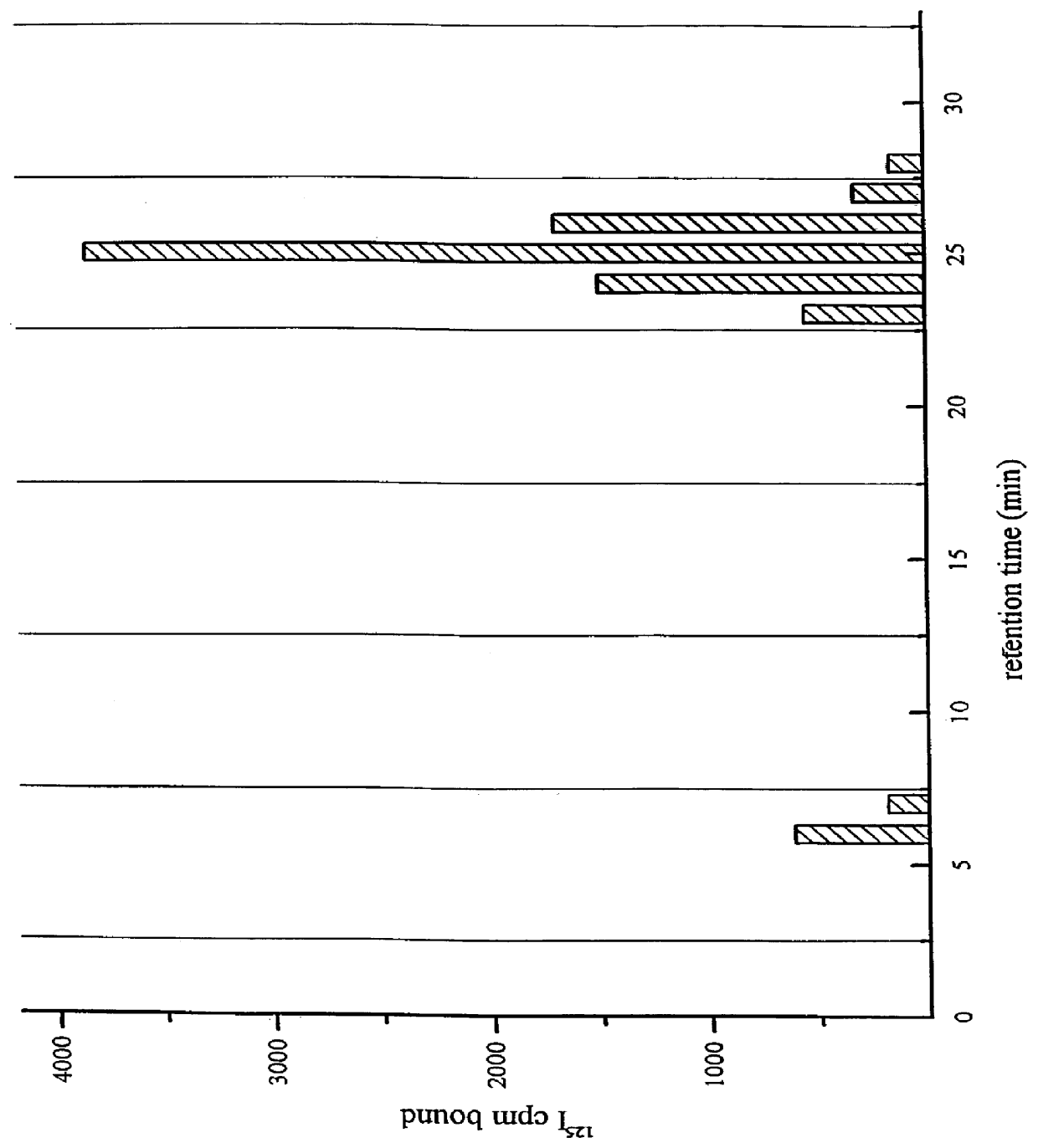
A method for extracting prion protein from a biological material, e.g., an animal tissue or product. In a specific example, abnormal prion protein is extracted from homogenized sheep brain with hexafluoro-2-propanol. The hexafluoro-2-propanol is separated from the aqueous brain preparation by increasing the ionic strength of the aqueous solution. Prion protein in the organic extract can be further purified, or the extract can be tested, e.g., by immunoassay, for the presence of prion protein, and more particularly abnormal prion protein. The extraction process permits testing for the presence of abnormal prior protein, e.g., for diagnosis of transmissible spongiform encephalopathies (TSE).
Owner:US SEC AGRI
Detection of transmissible spongiform encephalopathies
InactiveUS6033858ASugar derivativesMicrobiological testing/measurementSpiroplasmaTransmissible mink encephalopathy
Provided is a method of detecting transmissible spongiform encephalopathies. The method comprises: selecting a sample from a subject to determine whether the subject has a transmissible spongiform encephalopathy; and detecting spiroplasma-specific 16S rDNA indicative of transmissible spongiform encephalopathies in the sample. The spiroplasma-specific 16S rDNA is preferably detected by contacting the sample with a pair of oligonucleotide primers under polymerase chain reaction conditions and detecting the resulting polymerase chain reaction product, wherein each of the pair of the oligonucleotide primers is complementary to spiroplasma-specific 16S rDNA indicative of transmissible spongiform encephalopathies. Further provided is an oligonucleotide having a nucleotide sequence complementary to spiroplasma-specific 16S rDNA indicative of transmissible spongiform encephalopathies; as well as an oligonucleotide having a nucleotide sequence specific to spiroplasma-specific 16S rDNA indicative of transmissible spongiform encephalopathies.
Owner:BASTIAN FR O
Rapid prion-detection assay
Owner:PRION DEVAL LAB
Prion inhibiting peptides and derivatives thereof
InactiveUS20050181998A1Nervous disorderTetrapeptide ingredientsTransmissible spongiform encephalopathyProteinaceous infectious particle
Short peptides and derivatives or analogs thereof for the treatment or prevention of transmissible spongiform encephalopathies, in particular CJD are herein described. These peptides and / or their derivatives have been designed to block the conformational changes that occur in the prion protein (PrP) and which are implicated in the pathogenesis of transmissible spongiform encephalopathies as well as to dissolve the fibrillar deposits already formed
Owner:LAB SERONO SA
Modulation of prion expression
ActiveUS20110269818A1Lower Level RequirementsDownsized mannerOrganic active ingredientsNervous disorderHuntingtons choreaScrapie Virus
Disclosed herein are compounds and methods for decreasing PrP and preventing, ameliorating, or treating a prion disease or conformational neurodegenerative disorder, in an individual in need thereof. Examples of disease conditions that can be ameliorated with the administration of antisense compounds targeted to PrP include Creutzfeldt-Jakob disease (CJD); variant Creutzfeldt-Jakob Disease (vCJD); Gerstmann-Straussler-Scheinker syndrome; fatal familial insomnia; kuru; Bovine Spongiform Encephalopathy (BSE), e.g. “mad cow disease”; Chronic Wasting Disease (CWD); scrapie; transmissible mink encephalopathy; feline spongiform encephalopathy; ungulate spongiform encephalopathy; Alzheimer's disease; Parkinson's disease; Huntington's disease; and Amyotrophic Lateral Sclerosis (ALS).
Owner:IONIS PHARMA INC
Immunological assay for spongiform encephalopathies
InactiveUS20010010918A1Quick checkMethod is fastChemiluminescene/bioluminescenceImmunoglobulins against animals/humansAntibodySpongiform encephalopathy
Owner:OCONNOR MICHAEL
Use of prion conversion modulating agents
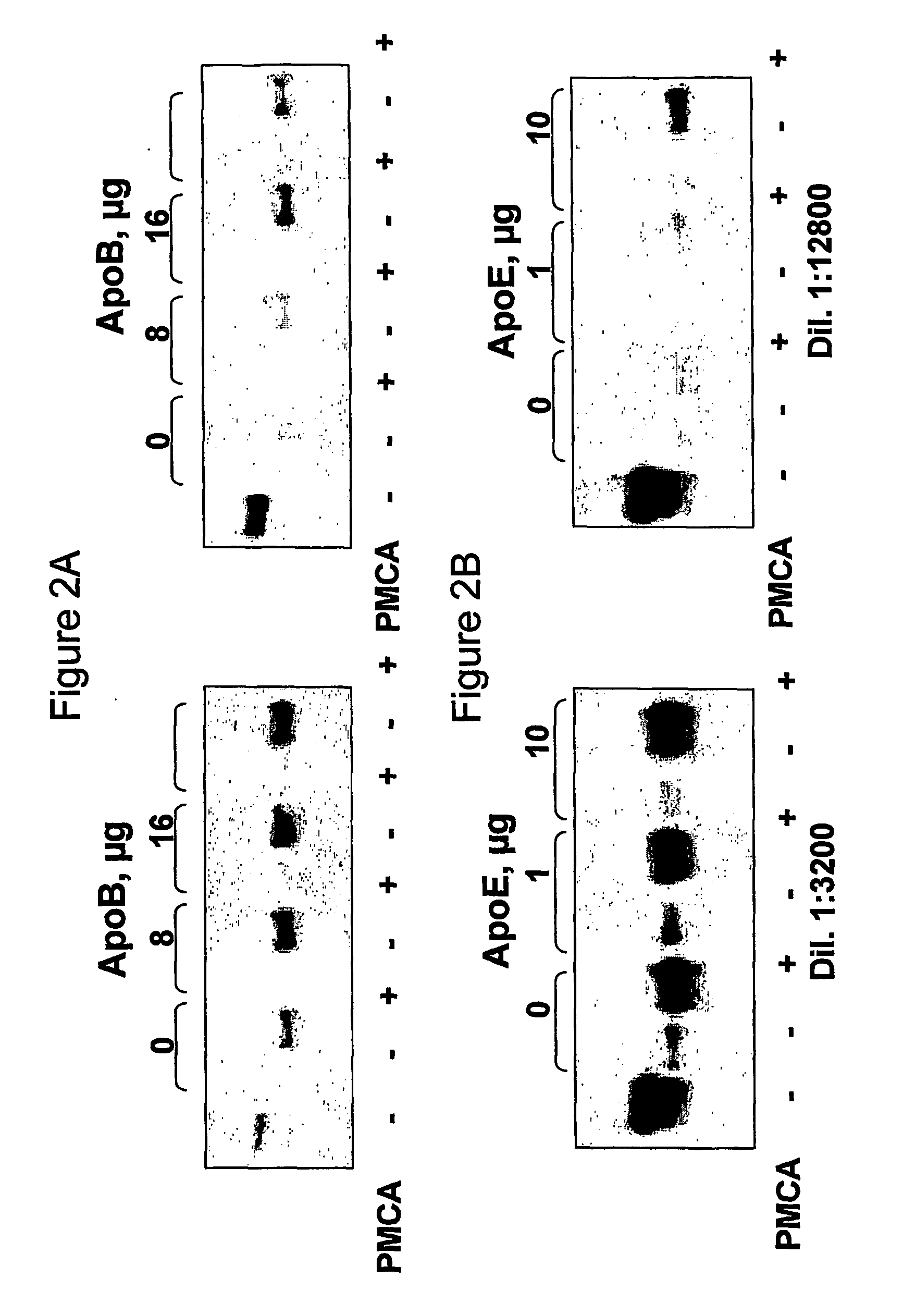
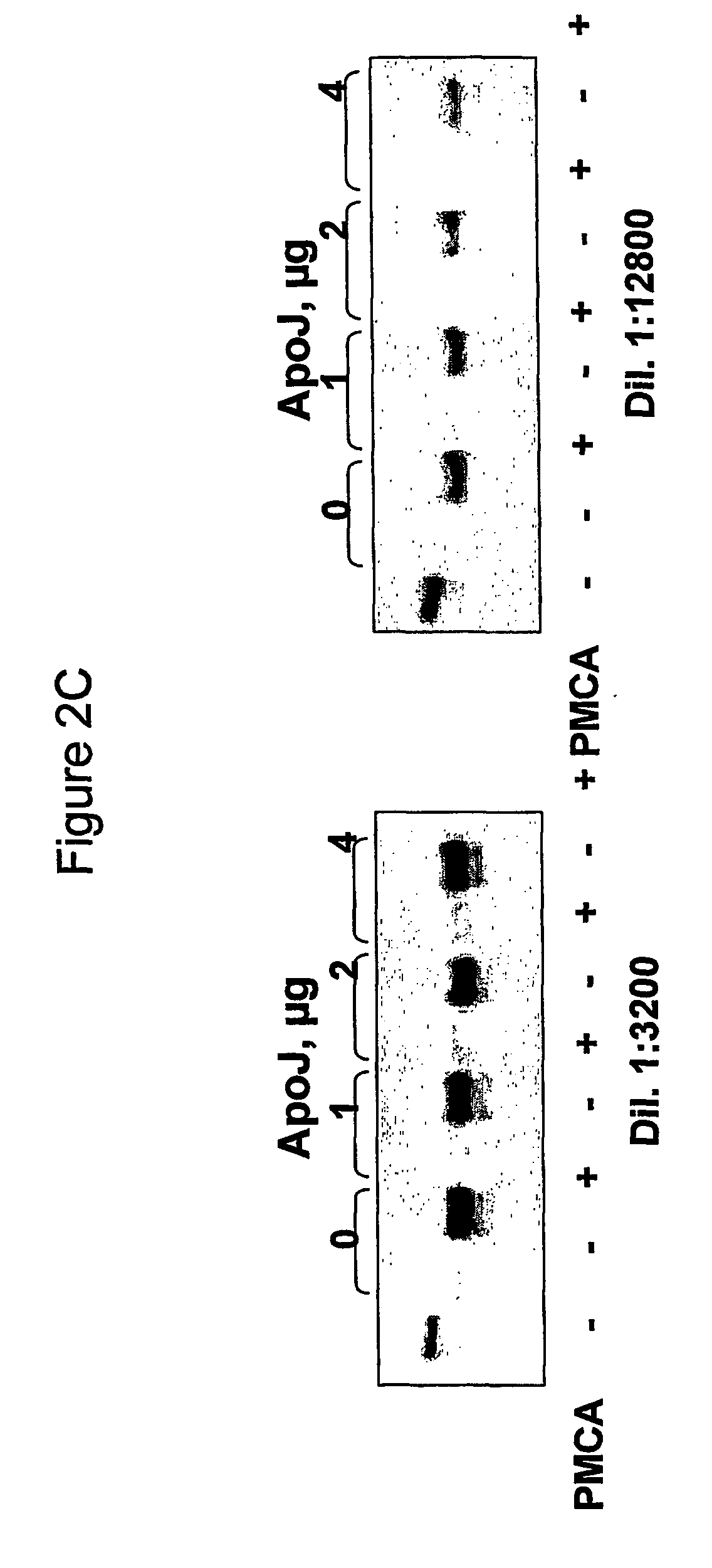
The use of Apolipoprotein B, Apolipoprotein E, fragments and mimetics thereof is provided for diagnostic, detection, prognostic and therapeutic applications In prion diseases. More specifically, the invention provides the use of Apolipoprotein B or fragments thereof for modulating or identifying modulators of the prion protein replication which are implicated in the pathogenesis of transmissible spongiform encephalopathics and other prion diseases.
Owner:MERCK SERONO SA
Diagnostic method for transmissible spongiform encephalopathies
InactiveUS20040171026A1Microbiological testing/measurementDisease diagnosisMass Spectrometry-Mass SpectrometryBody fluid
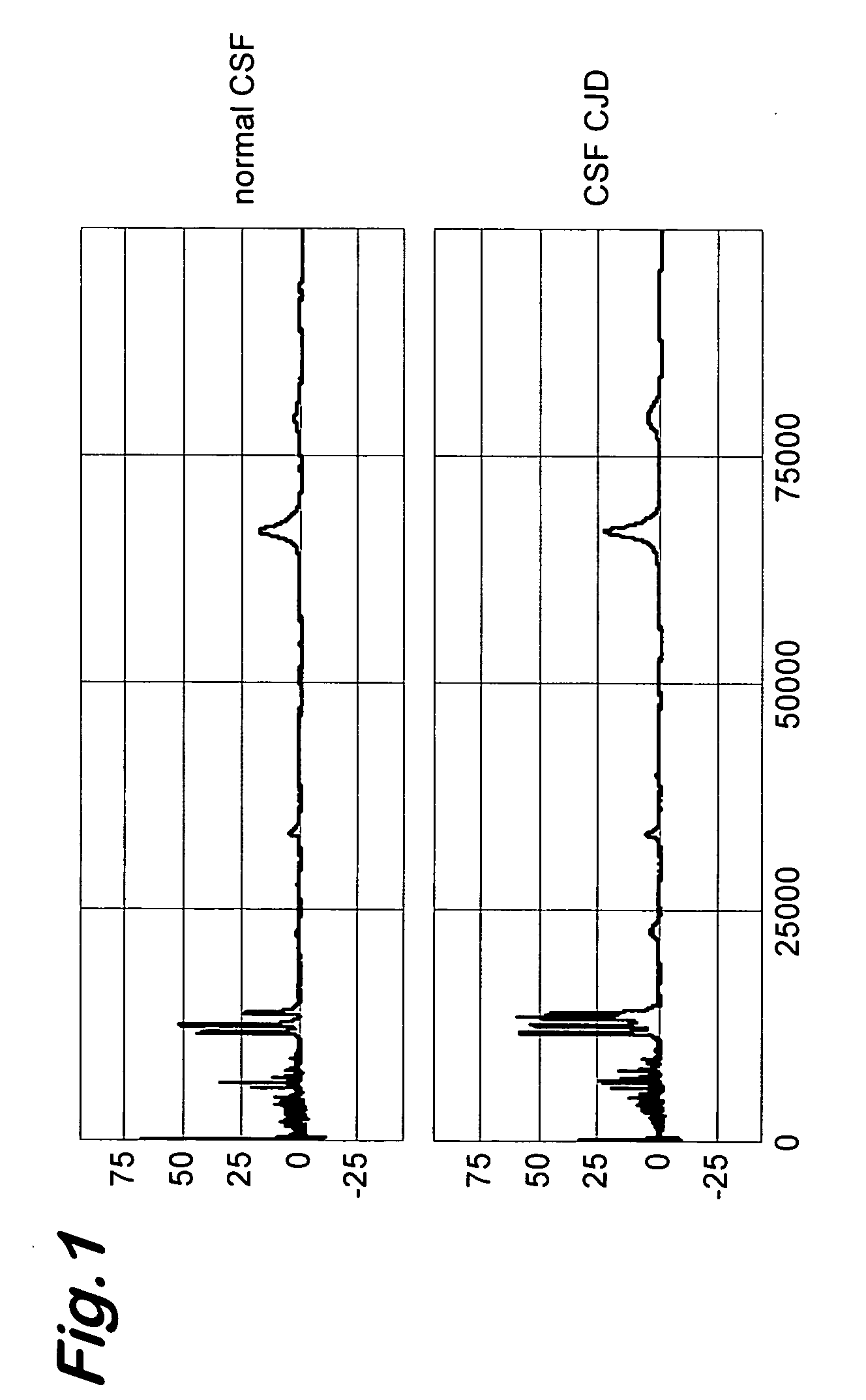
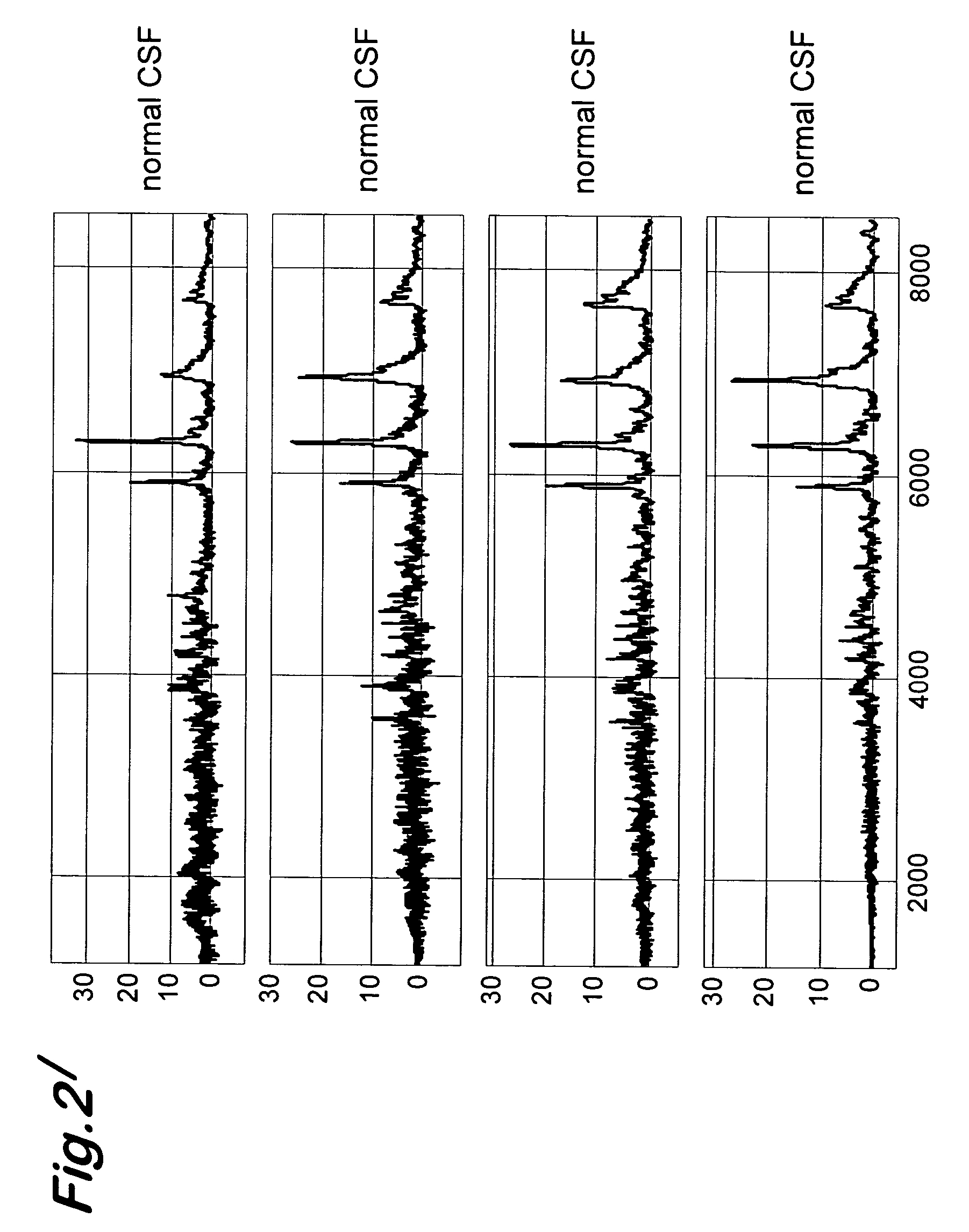
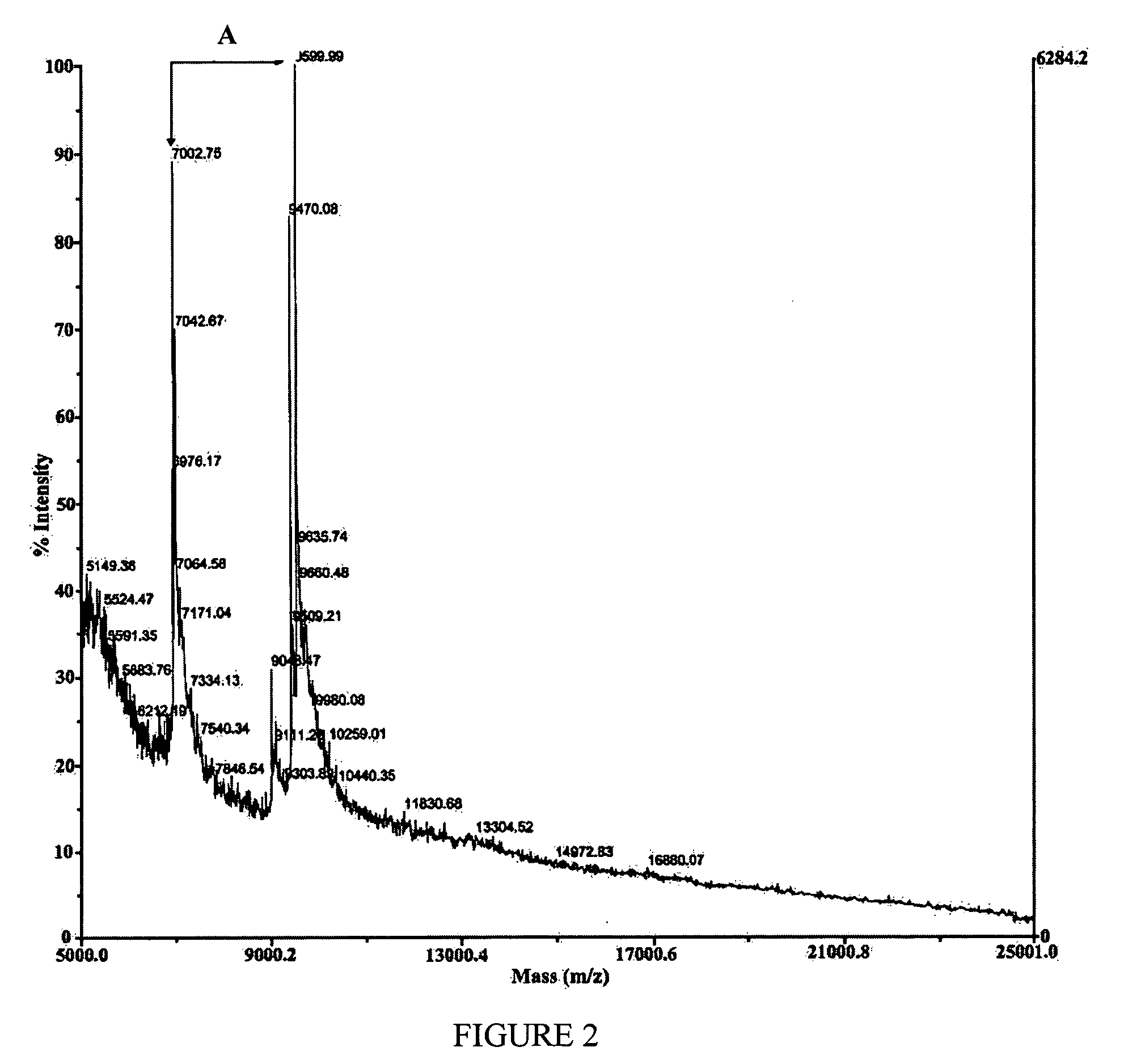
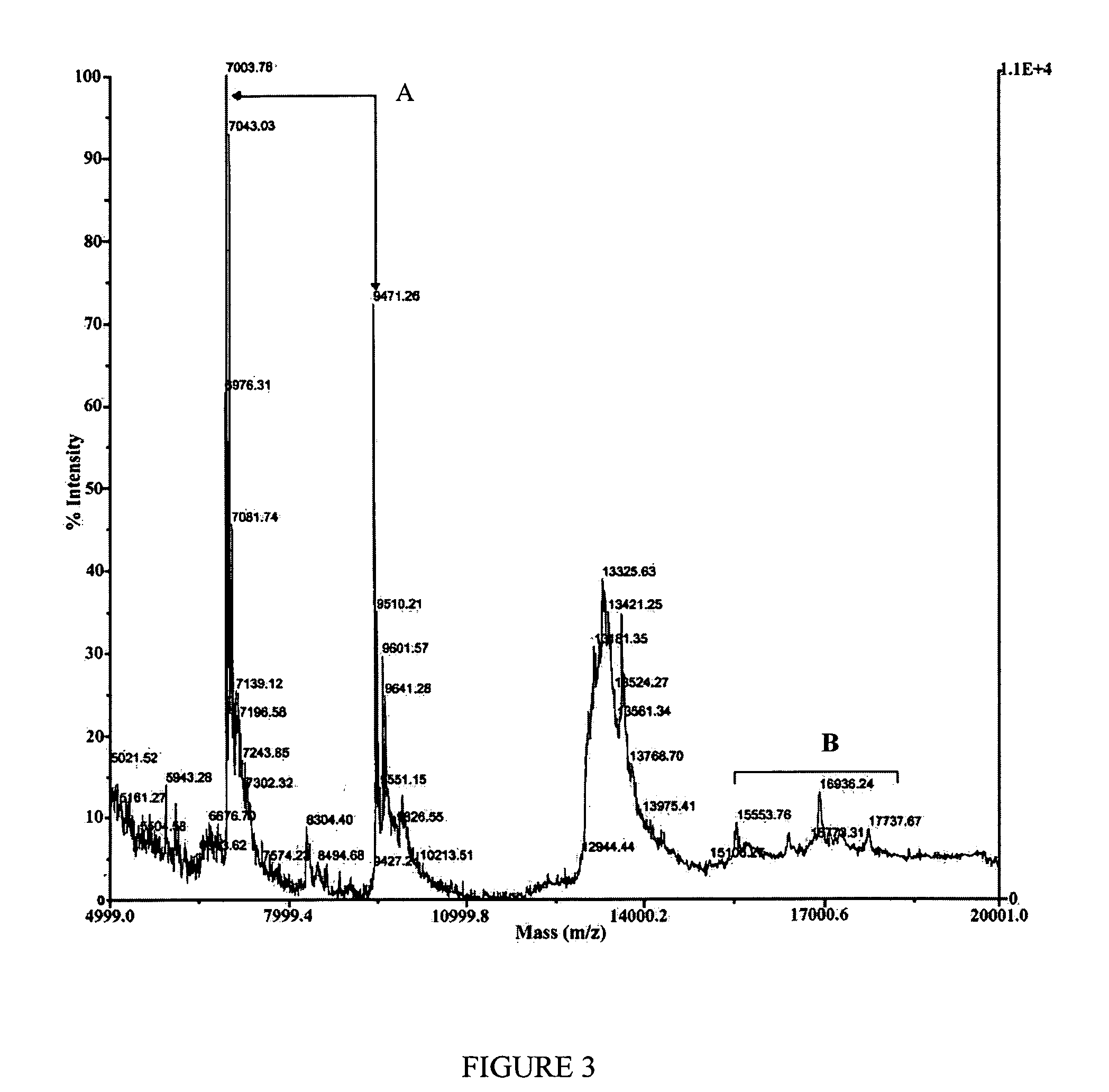
Transmissible spongiform encephalopathy (TSE) is diagnosed in a subject by using mass spectrometry to observe a polypeptide in a sample of body fluid taken from the subject. The polypeptide is differentially contained in the body fluid of TSE-infected subjects and non-infected subjects, and has a molecular weight in the range of from 1000 to 100000.
Owner:PROTEOME SCINECES
Process for detecting PrP using a macrocyclic adjuvant ligand
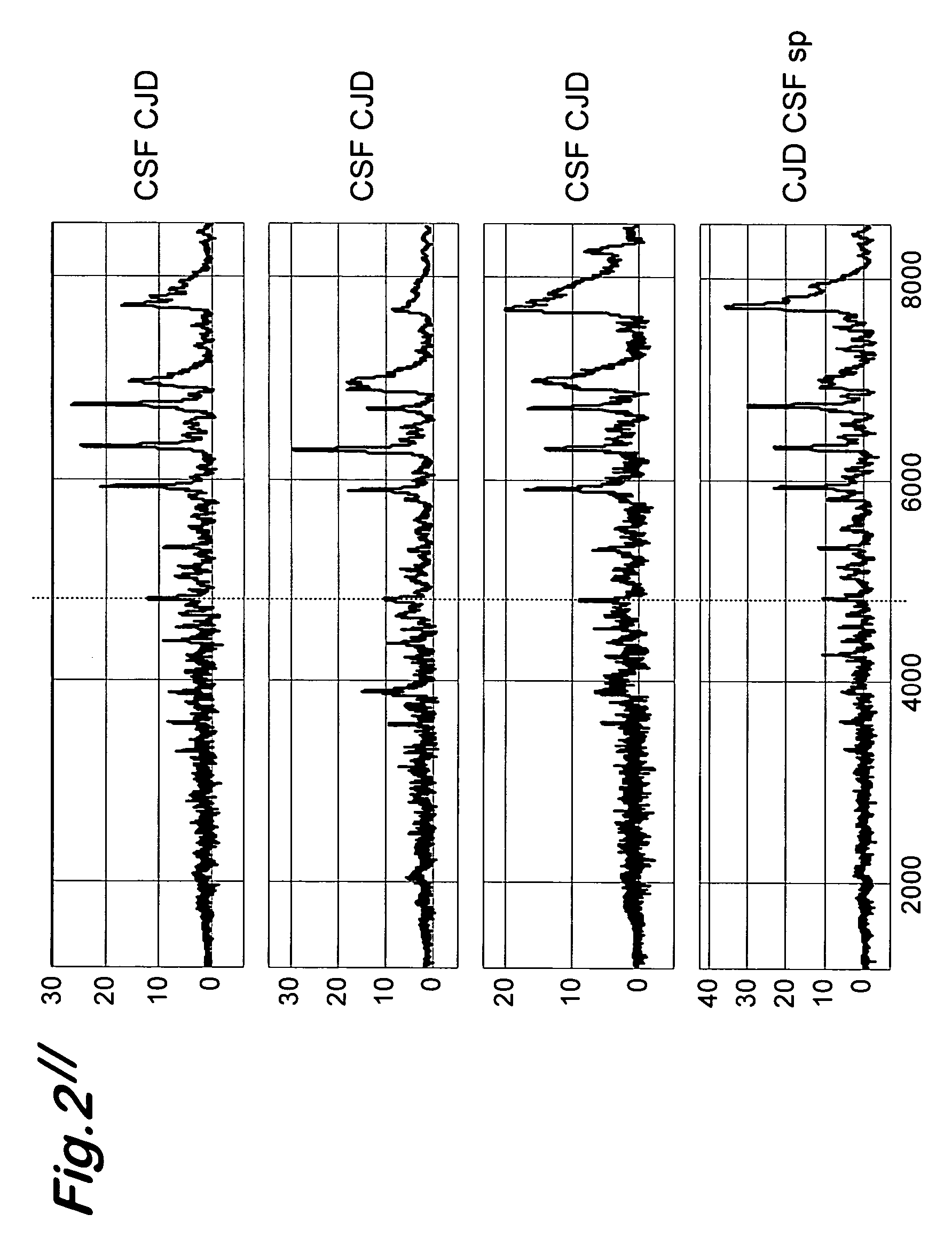
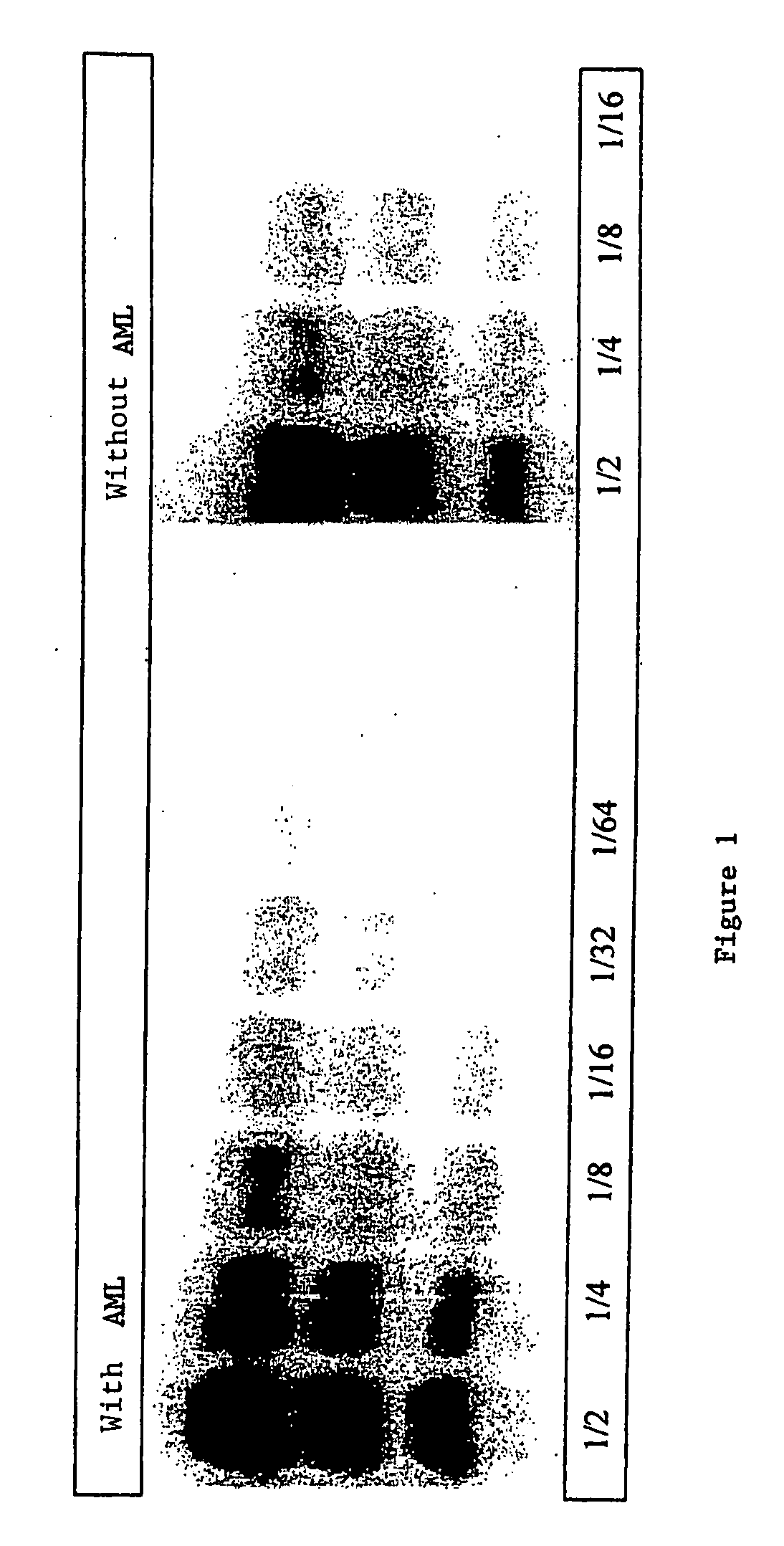
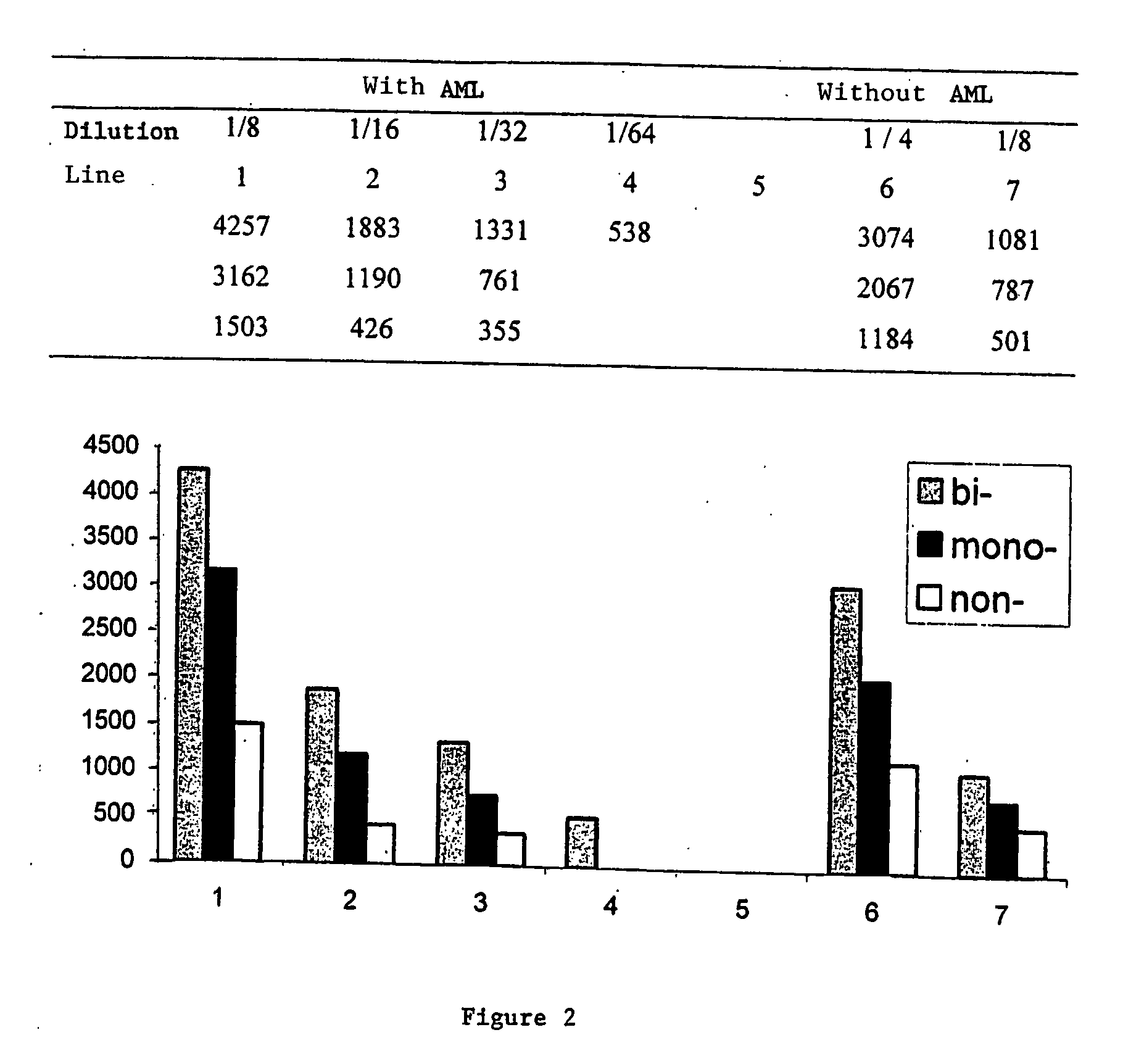
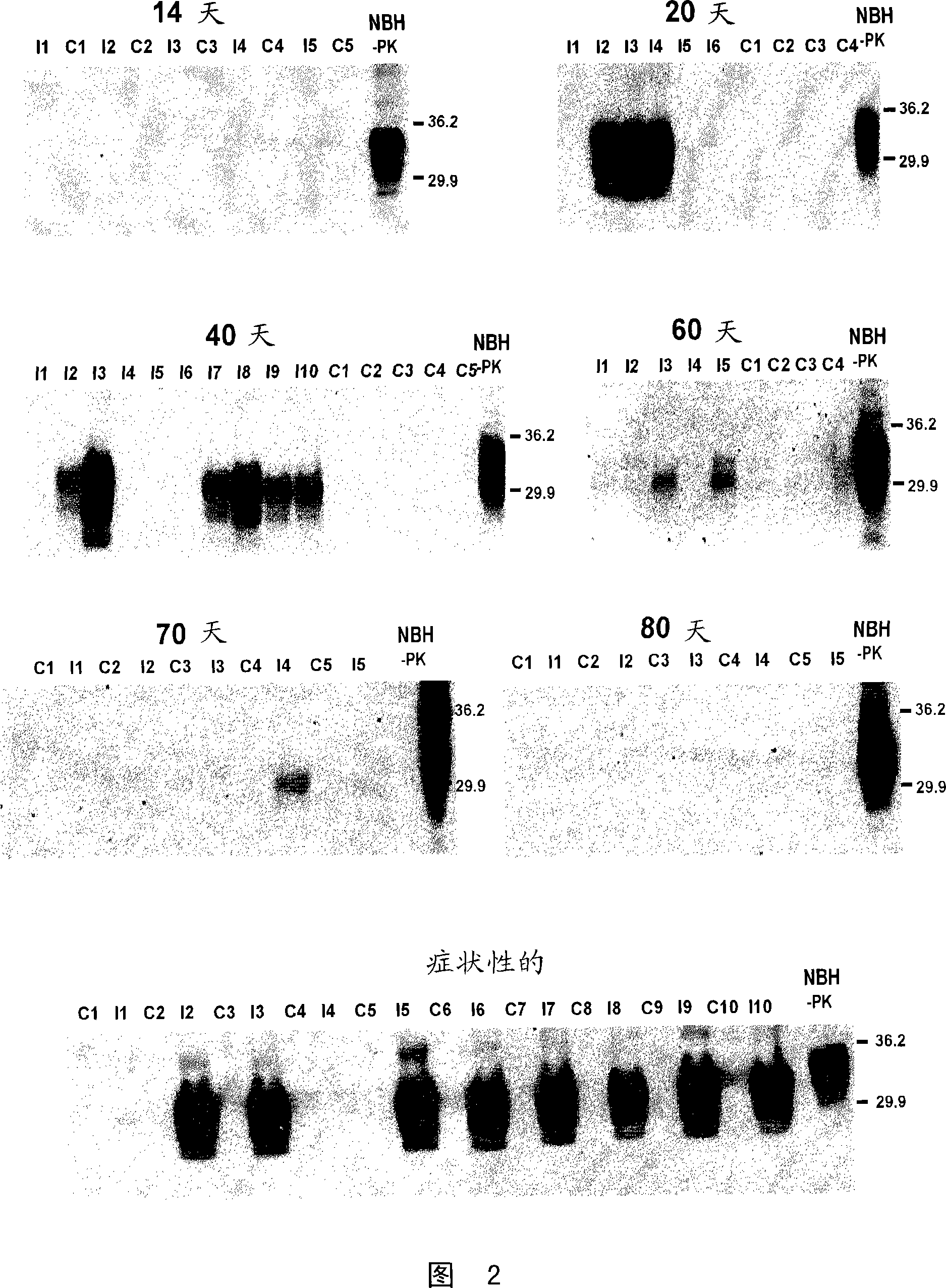
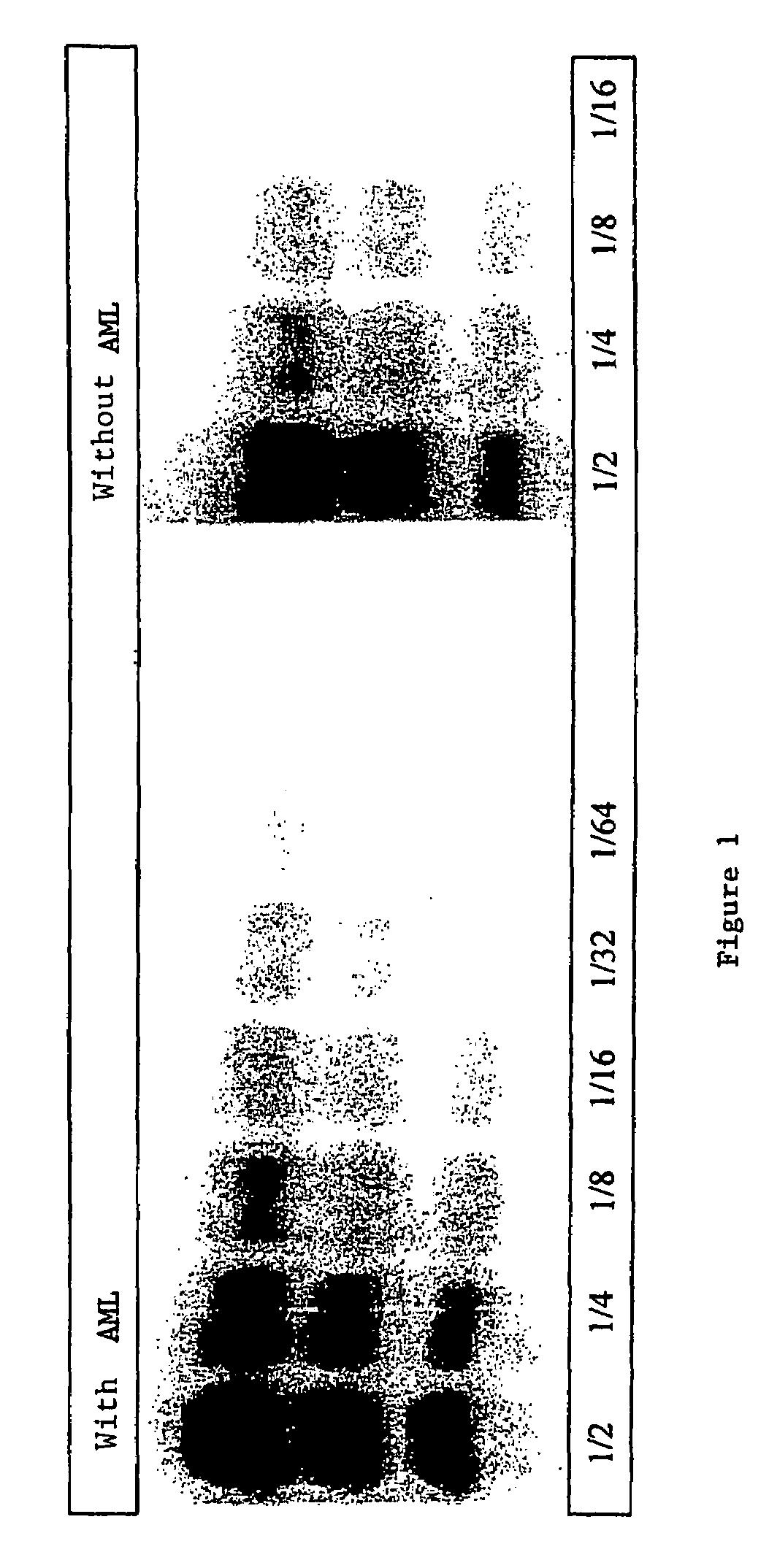
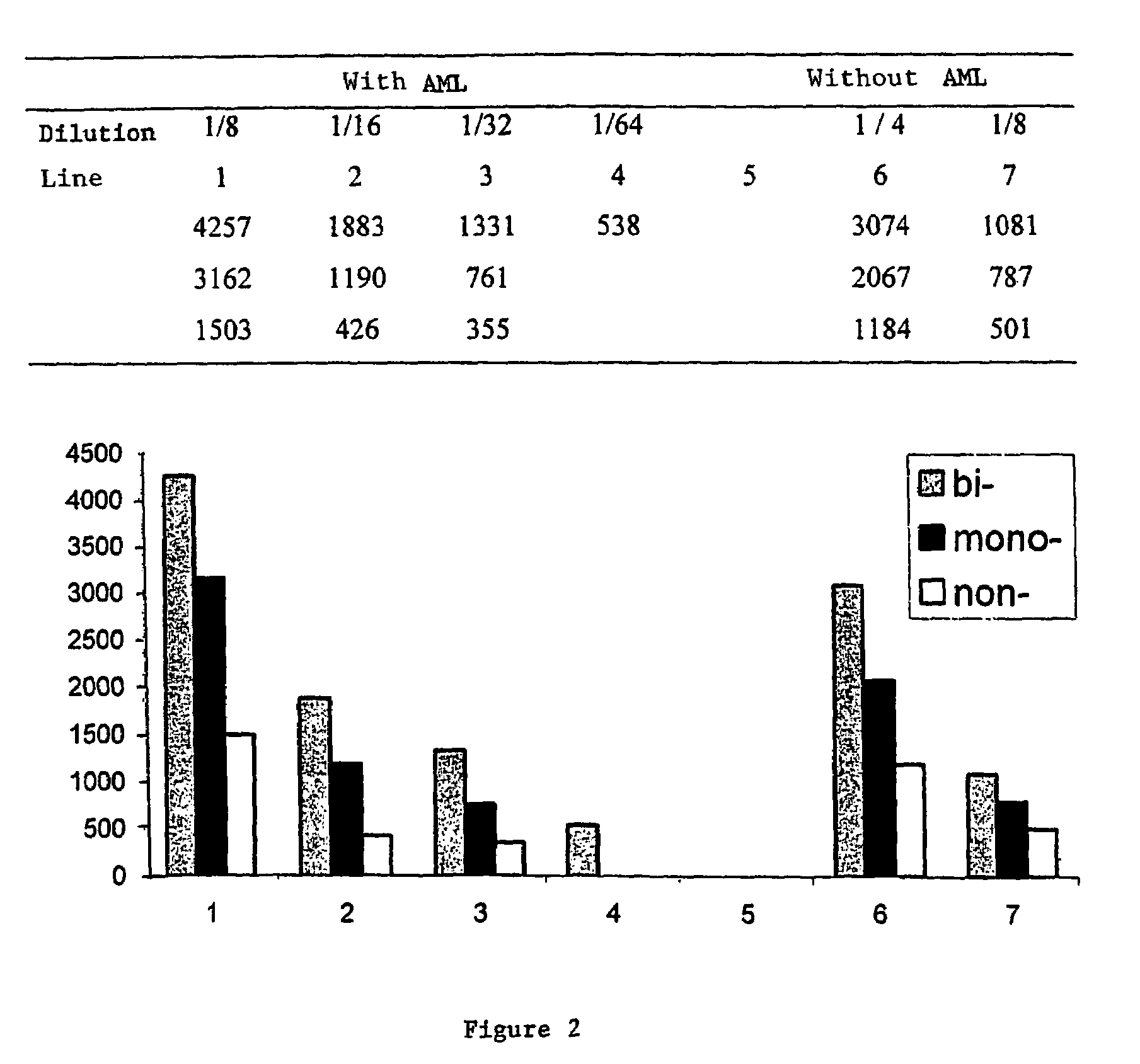
A process for detecting the forms of the prion pathogens responsible for subacute, transmissible, spongiform encephalopathies, including a macrocyclic adjuvant ligand (AML), free or bound to a support, that is added to a biological sample capable of containing PrPsc, the resulting suspension then being reacted with an anti-PrPsc antibody, and the presence of PrP is then detected.
Owner:AGENCE FRE DE SECURITE SANITAIRE DES ALIMENT +3
Modulation of prion expression
ActiveUS8669102B2Organic active ingredientsNervous disorderHuntingtons choreaFatal familial insomnia
Owner:IONIS PHARMA INC
Method for the Detection of Disease-Related Prion
InactiveUS20080220447A1Disease diagnosisBiological testingBiologyTransmissible spongiform encephalopathy
Methods for the detection of the disease associated conformation of the prion protein as an indication of transmissible spongiform encephalopathies (TSEs), including preclinical detection of infected live animals and humans, and post-mortem detection methods are disclosed. In one aspect, the tissue or body fluid sample of a test subject is contacted with an antibody that binds only the disease related conformation of the prion protein under non-denaturing conditions.
Owner:PRIONICS
Method for the detection of proteins of animal origin in complex mixtures
InactiveUS20050118720A1Avoid spreadingLow impurity contentNervous disorderComponent separationHigh concentrationLiquid chromatography mass spectroscopy
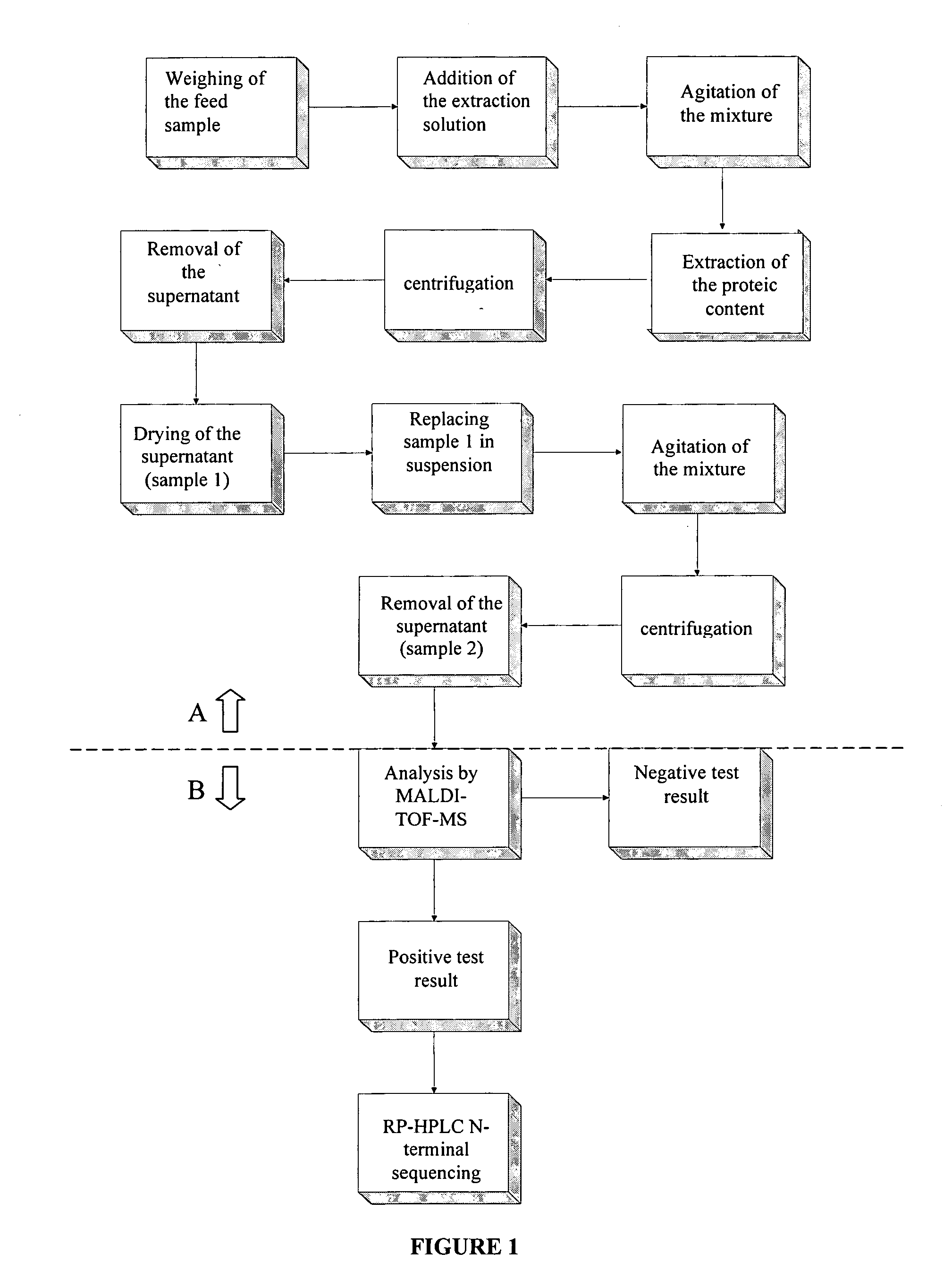
The purpose of the present invention is to evaluate the quality of feed for ruminants and consequently, avoid the transmission of TSEs through the detection of animal proteins in these foods. This purpose is embodied in the form of a method for detecting proteins of animal origin in complex mixtures comprising the stages of: (i) extraction of the proteic matter in high concentration from a sample of the initial complex mixture in a manner as to substantially remove all interferents; (ii) preparation of the matrix-analyte in a manner as to maintain low levels of impurities and an adequate matrix-analyte molar rate; (iii) analysis of the material obtained in the prior stage by MALDI-TOF mass spectrometry; (iv) optionally, fractionation of the samples or isolation of the components by RP-HPLC and identification of the components by means of automatic sequencing of the N-terminal region and sequencing of its peptidic fragments by liquid chromatography coupled to mass spectrometry (LC / MS / MS). The present invention also contemplates the use of this method in the detection of proteins of animal origin in feed for ruminants, which permits the interruption of transmission of Transmittable Spongiform Encephalopathies, and more particularly Bovine Spongiform Encephalopathies (BSE).
Owner:EMPRESA BRASILEIRA DE PESQUISA AGROPECUARIA EMBRAPA
Methods for rapid screening of mad cow disease and other transmissible spongiform encephalopathies
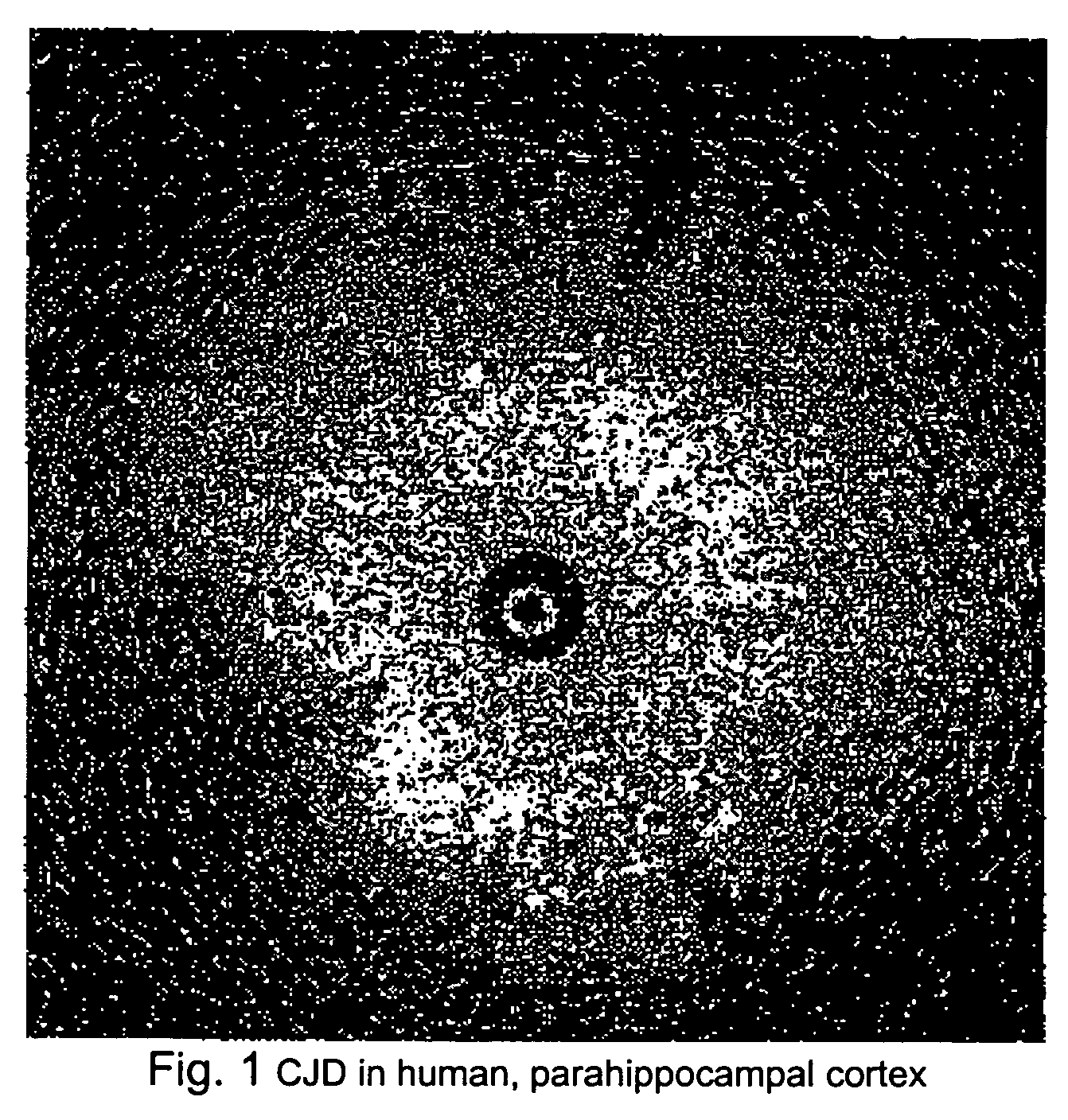
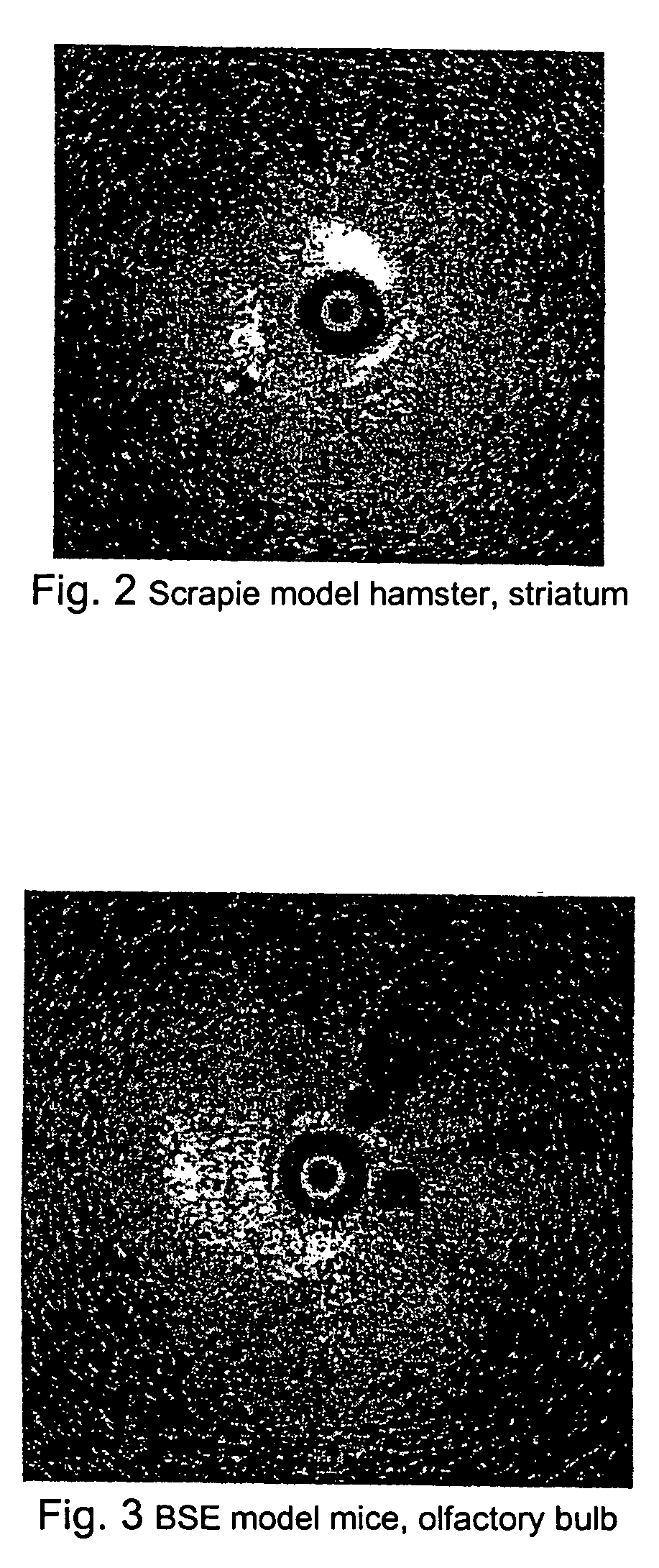
Methods for diagnosing altered neuropathology in an animal are disclosed, wherein said methods comprise imaging brain, spinal cord, or other neural tissue of the animal, analyzing the appearance of the tissue, and determining whether the appearance of the tissue is altered relative to corresponding unaltered tissue. Also disclosed are methods for diagnosing spongiform encephalopathies in an animal, wherein said methods comprise imaging brain, spinal cord, or other neural tissues of the animal, analyzing the appearance of vacuoles in the tissue, and determining whether the appearance of the vacuoles in the tissue is altered relative to corresponding spongiform encephalopathy-free tissue. Also disclosed are automated methods for diagnosing altered neuropathy and spongiform encephalopathies.
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS +1
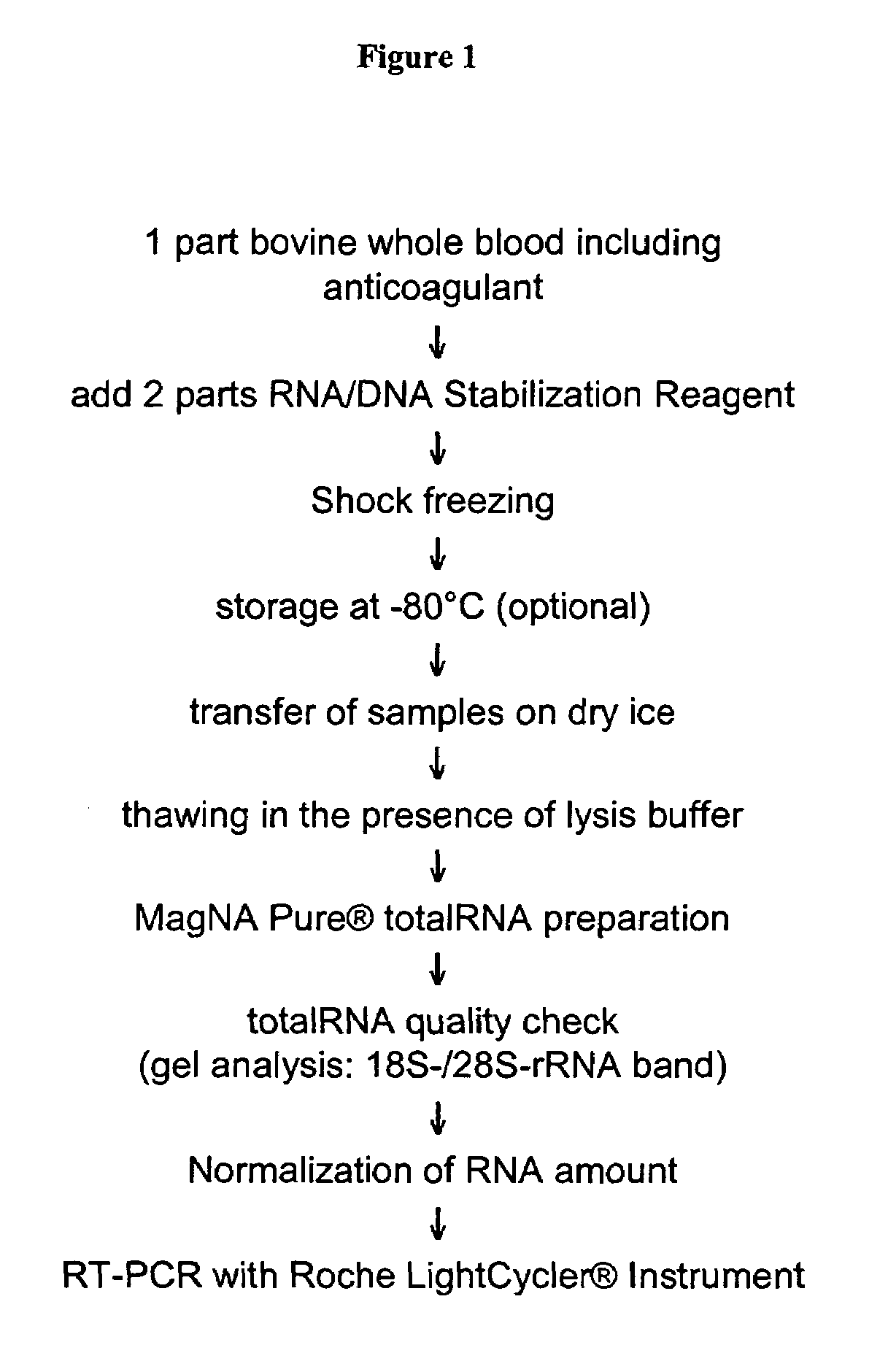
Nucleic acid preparation from whole blood for use in diagnosis of transmissible spongiform encephalopathy
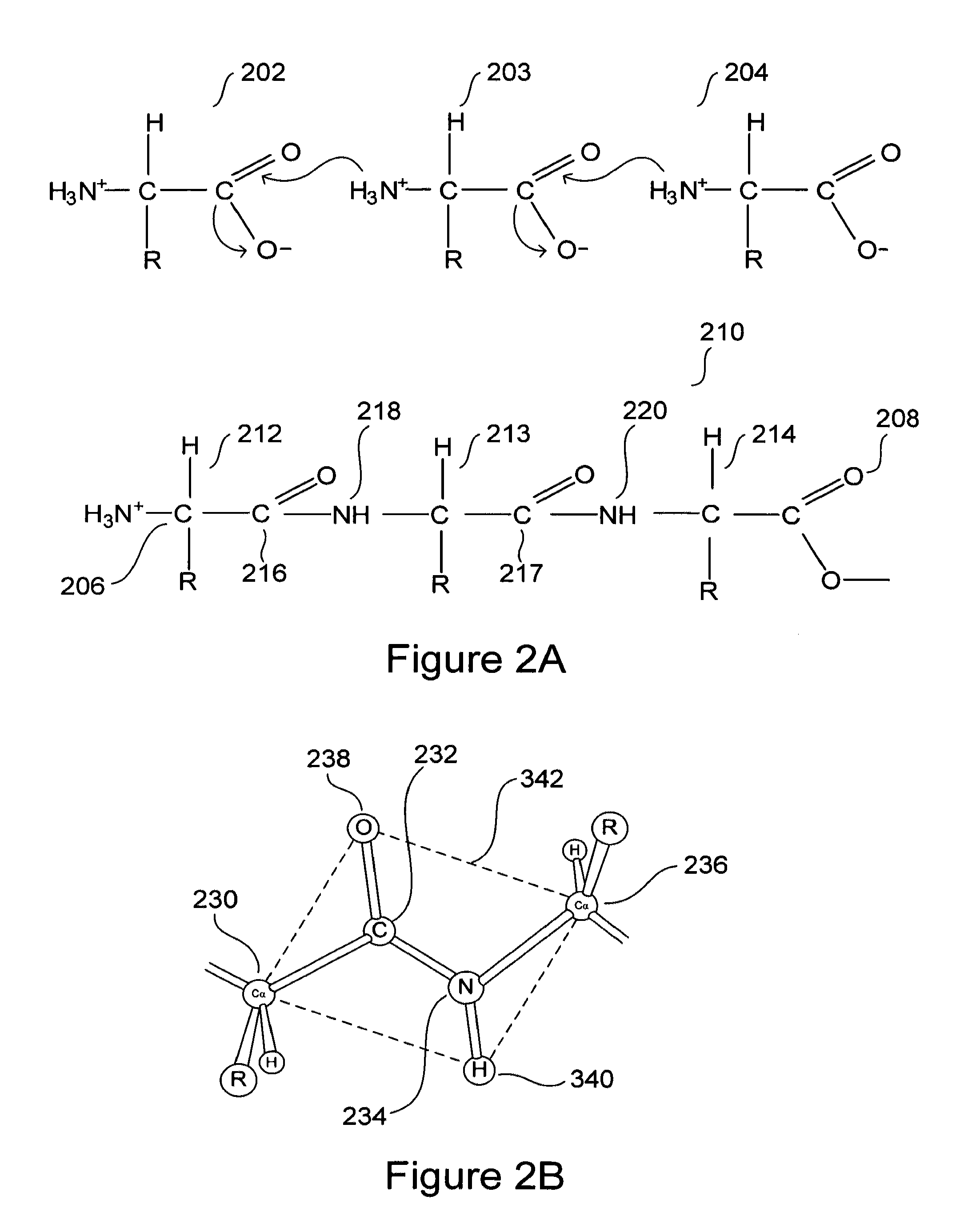
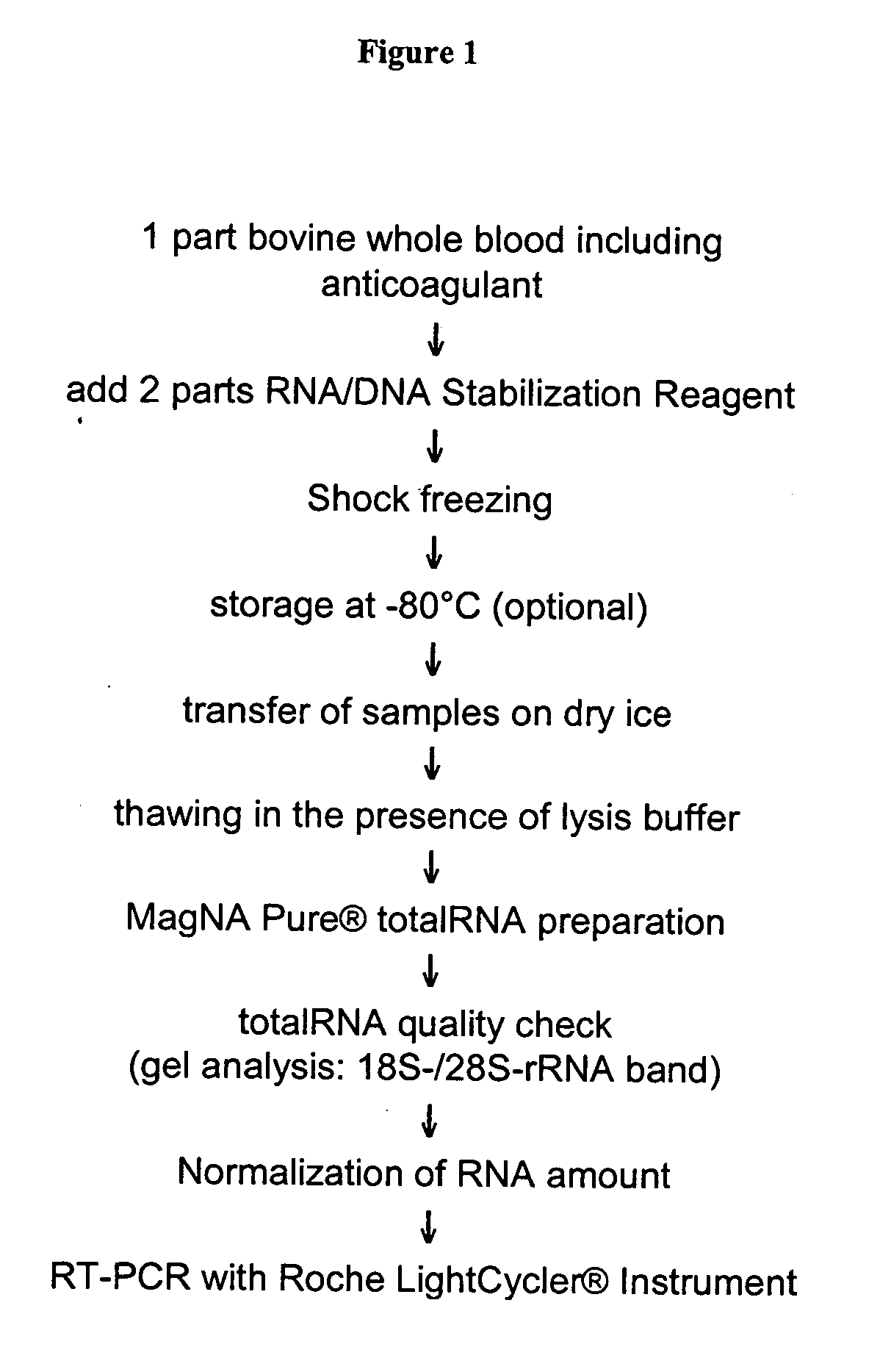
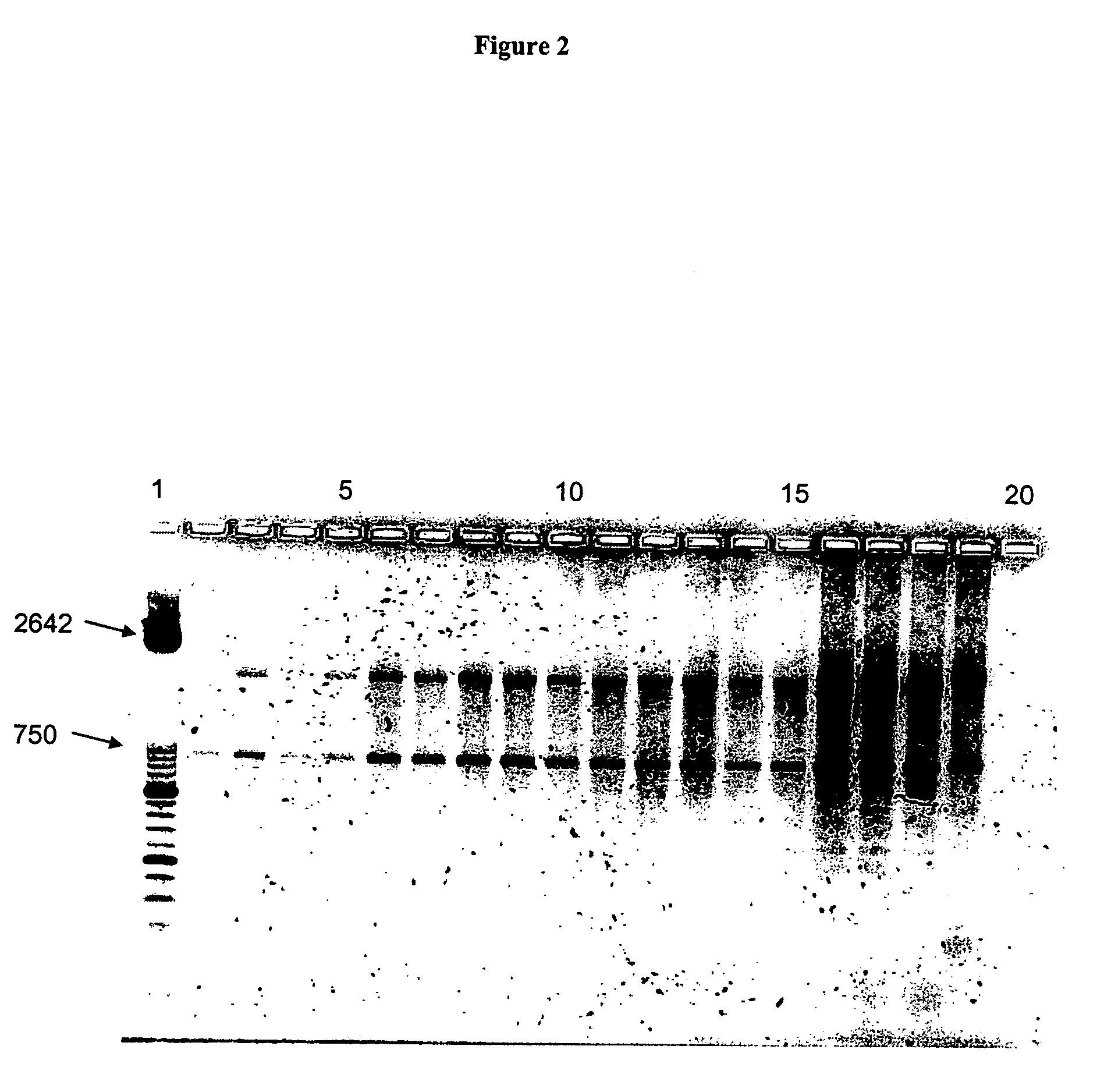
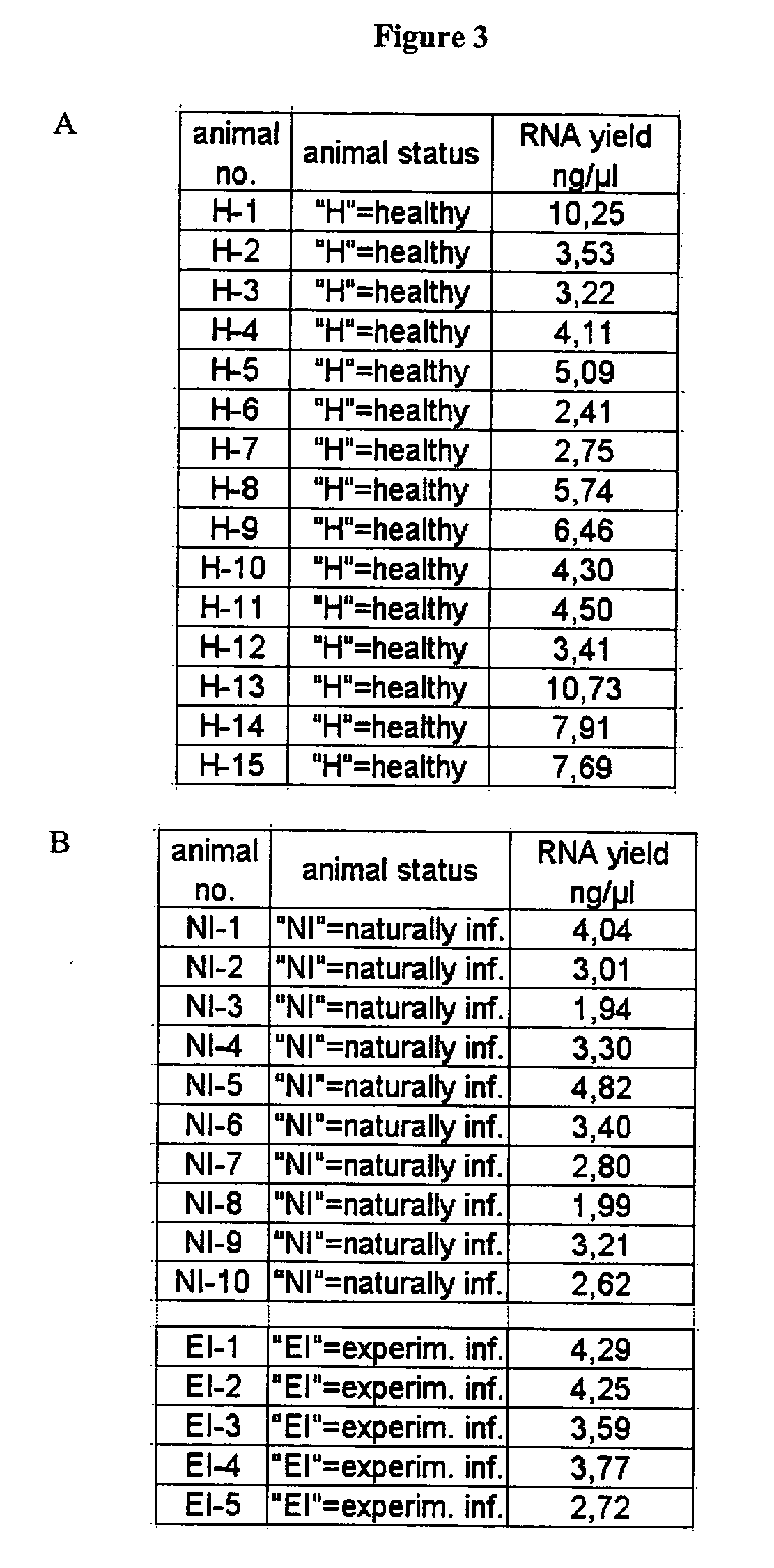
The present invention deals with a method of preparing nucleic acids, particularly RNA, from a whole blood sample. The nucleic acids purified by the method of the invention are particularly suited for detection of nucleic acid marker molecules. Preferred are markers for the diagnosis of transmissible spongiform encephalopathy (TSE). Such diagnosis is based on the detection, by means of real-time PCR, of certain mRNAs of the TSE-infected organism. Said mRNAs specifically originate as splicing variants and are isolated from whole blood by the method of the invention.
Owner:ROCHE DIAGNOSTICS OPERATIONS INC
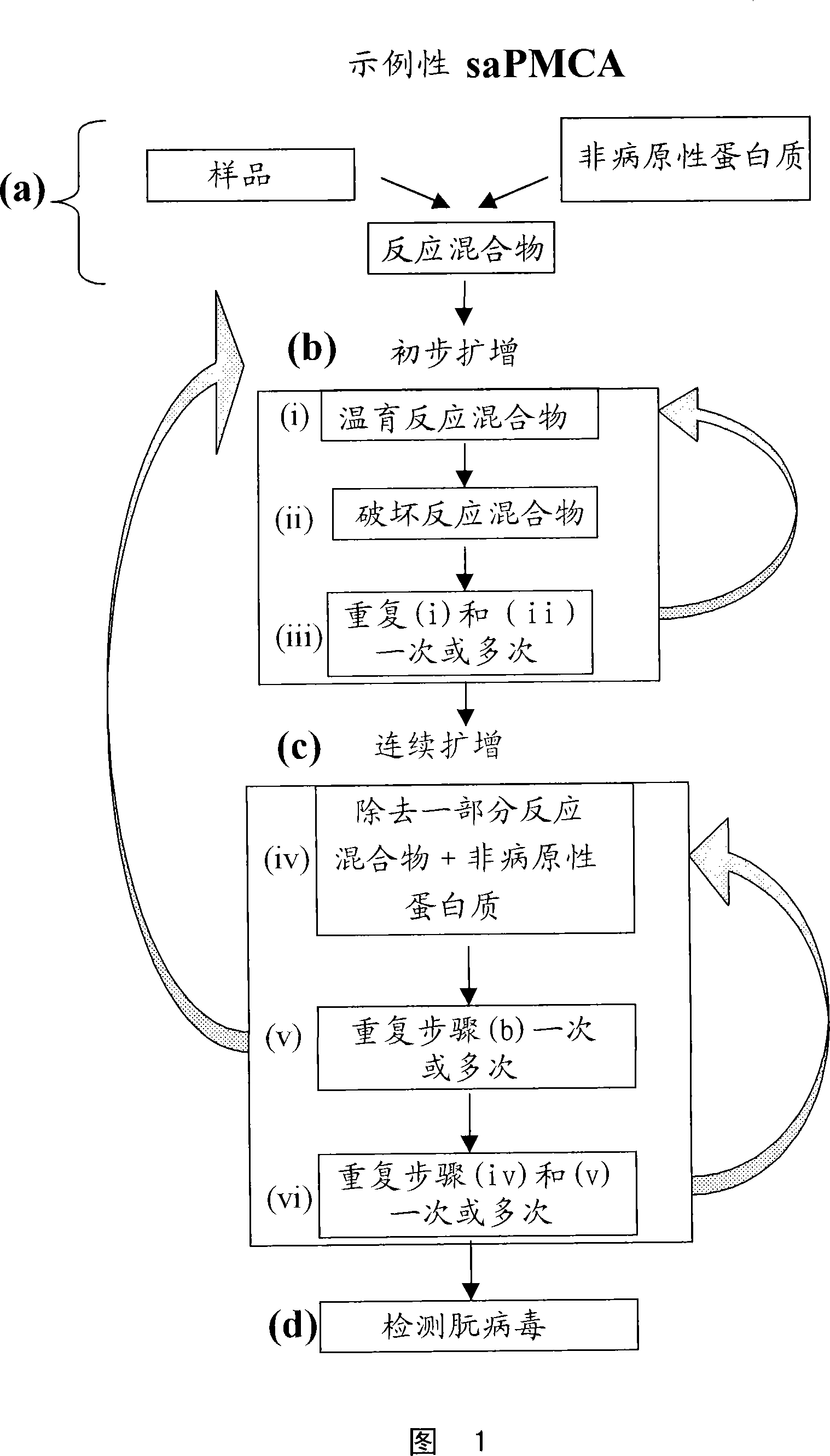
Ultrasensitive detection of prions by automated protein misfolding cyclic amplification
A highly sensitive method for detecting prions in a sample is provided. These methods can be used to diagnose prion-mediated transmissible spongiform encephalopathy, such as bovine spongiform encephalopathy, Creutzfeldt-Jakob disease, scrapie or chronic wasting disease. In particular, methods for continuous automated cyclic expansion of prions are disclosed. The method is fast and highly sensitive, making it ideal for high-throughput testing.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Process for detecting PrP using a macrocyclic adjuvant ligand
InactiveUS7217530B2Microbiological testing/measurementEnzymologyAdjuvantProteinaceous infectious particle
A process for detecting the forms of the prion pathogens responsible for subacute, transmissible, spongiform encephalopathies, including a macrocyclic adjuvant ligand (AML), free or bound to a support, that is added to a biological sample capable of containing PrPsc, the resulting suspension then being reacted with an anti-PrPsc antibody, and the presence of PrP is then detected.
Owner:AGENCE FRE DE SECURITE SANITAIRE DES ALIMENT +3
Monoclonal antibody for resisting bovine prion protein and application thereof
InactiveCN101125890ASmall molecular weightGuaranteed specificityNervous disorderImmunoglobulins against animals/humansSingle-Chain AntibodiesGenetic engineering
The invention relates to a monoclonal antibody prepared with genetic engineering and the preparation method and usage thereof. Exactly speaking, the invention relates to monoclonal antibody mab-PrP102-6 of bovine prion protein PrP27-30 and .
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Antibodies for discrimination of prions
ActiveUS7399603B2Improve isolationMicrobiological testing/measurementImmunoglobulins against cell receptors/antigens/surface-determinantsTransmissible spongiform encephalopathyProteinaceous infectious particle
Owner:ORTHO-CLINICAL DIAGNOSTICS
Complexes comprising a prion protein and a peptidyl prolyl isomerase chaperone, and method for producing and using them
The present invention relates to the diagnosis of transmissible spongiform encephalopathies (TSEs). It especially teaches the production of a soluble prion protein (PrP)-chaperone complex and the advantageous use of such chaperone-PrP complex, especially in the detection of PrP in an immunoassay, as well as its use as and immunogen.
Owner:ROCHE DIAGNOSTICS GMBH
Medium composition for preparing botulinum toxin
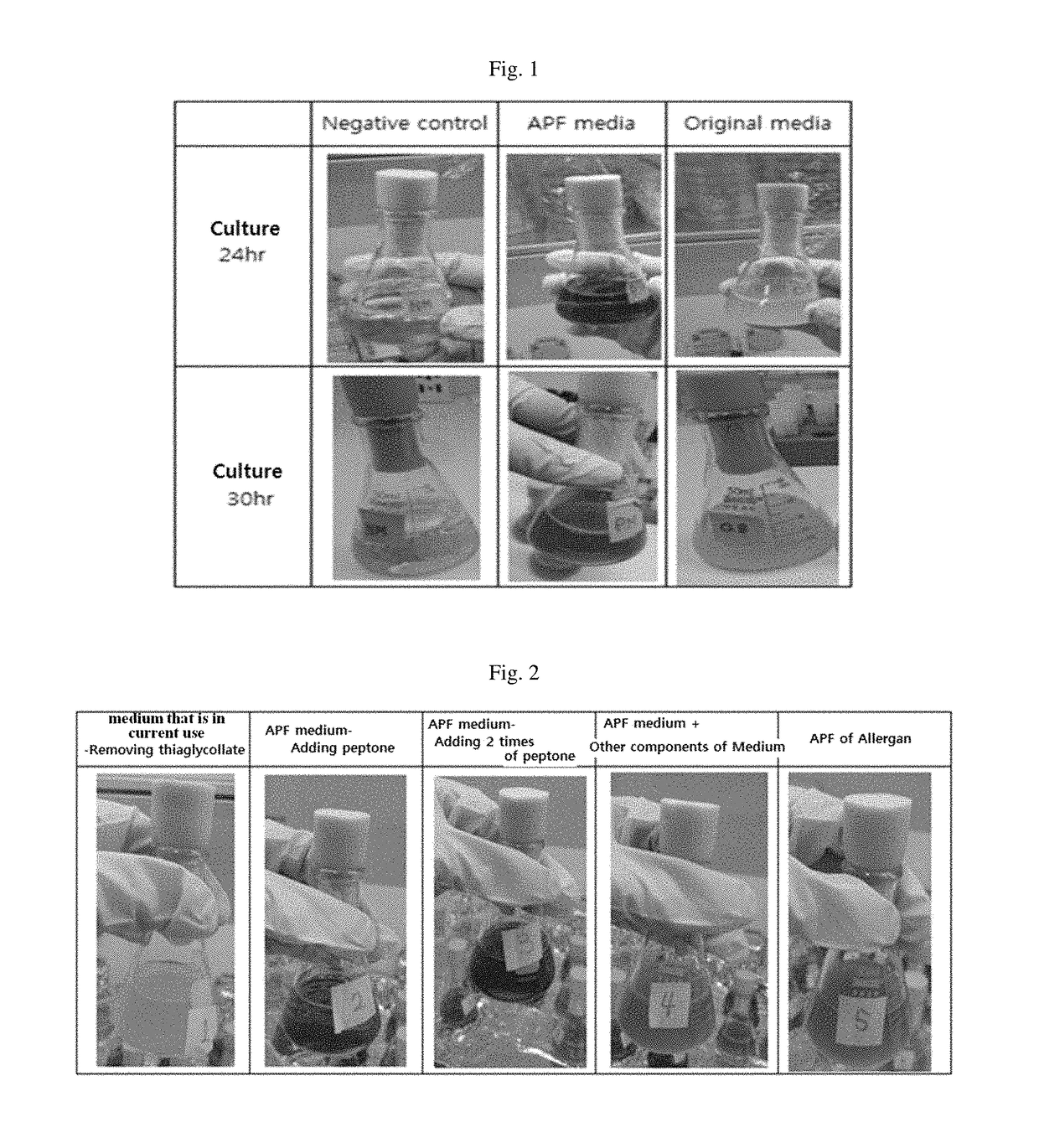
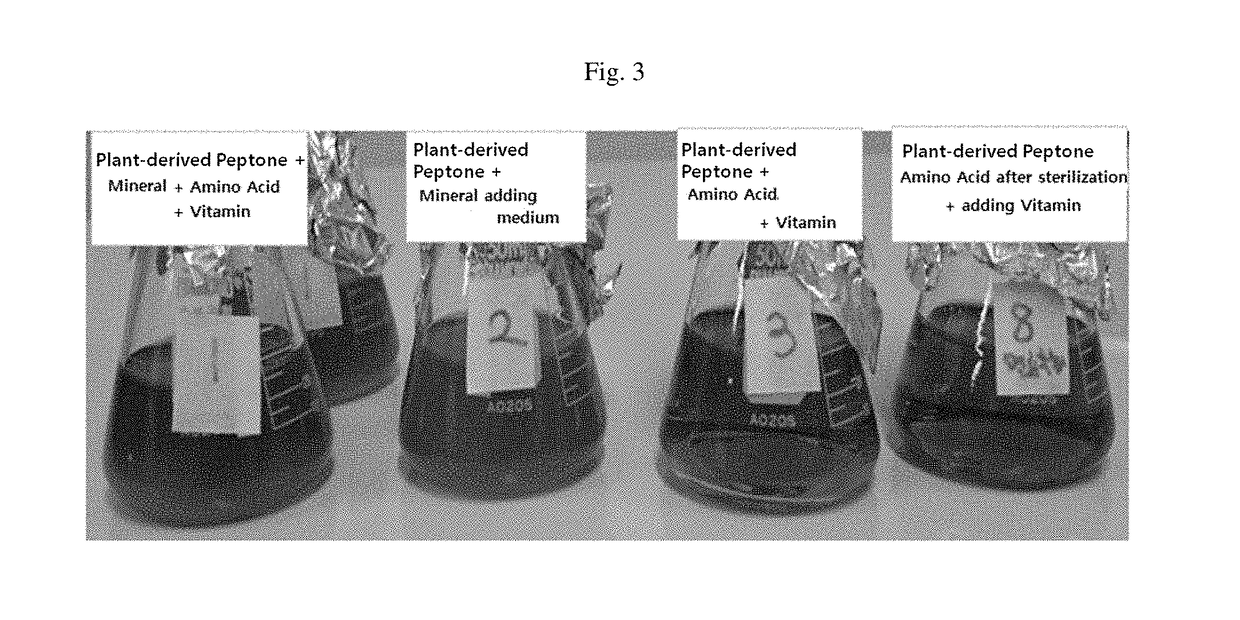
The present invention relates to a medium composition for production of botulinum toxin and, more particularly, to a medium composition for culture of Clostridium sp. capable of producing botulinum toxin. The medium composition of the present invention comprises at least one plant-derived peptone selected from the group consisting of a garden pea hydrolysate, a cotton seed hydrolysate and a wheat gluten hydrolysate. When the medium according to the present invention, which contains plant-derived peptones and minerals, is used for culture of Clostridium botulinum, the growth rate of the bacterium in the medium is about 1.5-2 times higher than that in the medium that is in current use. In addition, when botulinum toxin is produced by culturing the bacterium in the medium, infection with transmissible spongiform encephalopathy (TSE) or the like can be prevented by blocking introduction of animal-derived components.
Owner:DAEWOONG CO LTD
Method for chromatographic removal of prions
A method for removing a prion from a solution comprising the prion and at least one additional biomolecule, comprising directing the solution through an anion-exchange chromatography column under conditions that cause a gradient elution, whereby the prion is separated from at least one of the biomolecules, thereby causing said biomolecule to be collected in an eluate fraction that is distinct from an eluate fraction that includes the prion. In one embodiment, the gradient is a pH gradient, for example, a step gradient. The prion can be a causal agent for a spongiform encephalopathy, such as Creutzfeldt-Jakob disease, Gerstmann-Straussler-Scheinken syndrome, scrapie, or bovine spongiform encephalopathy.
Owner:HEMOGLOBIN OXYGEN THERAPEUTICS
Soluble Hybrid Prion Proteins And Their Use In The Diagnosis, Prevention And Treatment Of Transmissible Spongiform Encephalopathies
InactiveUS20080287348A1Improve permeabilitySlow onsetOrganic active ingredientsPeptide/protein ingredientsHybrid proteinTransmissible spongiform encephalopathy
The present invention relates to a soluble hybrid protein, comprising at least a first polypeptide sequence derived from a prion protein PrPc that is capable of binding a protein responsible for transmissible spongiform encephalitis (PrPSc), and a second polypeptide sequence (tag), wherein said hybrid protein does not comprise a functional membrane anchor moiety. Also, the present invention is directed to the use of said hybrid proteins for the diagnosis of transmissible spongiform encephalopathies. In addition, the present invention relates to the use of said hybrid protein, a nucleic acid encoding said hybrid protein, a vector, and / or a host cell comprising a nucleic acid encoding said hybrid protein for the preparation of a medicament for the prevention or treatment of transmissible spongiform encephalopathies (TSEs). Furthermore, the present invention is directed to a process for producing said hybrid proteins and it also relates to transgenic animals stably expressing said hybrid protein, the bone marrow of said transgenic animals and the use of said bone marrow for the treatment of transmissible spongiform encephalopathies (TSEs).
Owner:UNIV ZURICH
Medium composition for preparing botulinum toxin
The present invention relates to a medium composition for production of botulinum toxin and, more particularly, to a medium composition for culture of Clostridium sp. capable of producing botulinum toxin. The medium composition of the present invention comprises at least one plant-derived peptone selected from the group consisting of a garden pea hydrolysate, a cotton seed hydrolysate and a wheat gluten hydrolysate. When the medium according to the present invention, which contains plant-derived peptones and minerals, is used for culture of Clostridium botulinum, the growth rate of the bacterium in the medium is about 1.5-2 times higher than that in the medium that is in current use. In addition, when botulinum toxin is produced by culturing the bacterium in the medium, infection with transmissible spongiform encephalopathy (TSE) or the like can be prevented by blocking introduction of animal-derived components.
Owner:DAEWOONG CO LTD
Computationally designed inhibitors of amyloidosis
ActiveUS20090248379A1Inhibit aggregationOrganic active ingredientsNervous disorderAmyloid betaFibril
Embodiments of the present invention include methods and systems for designing inhibitors of amyloidosis in humans, domesticated animals, and wild animals as well as inhibitors of amyloidosis designed by the methods and systems. Methods and systems for designing inhibitors of amyloidosis are largely computational, in nature, and are directed to designing various types of polymers, small-molecule organic compounds, organometallic compounds, or non-chemical physical processes that can target the extended-α-strand and α-sheet regions of amyloidogenic protein and polypeptide intermediates in order to prevent aggregation of those intermediates into protofibrils and fibrils that, in turn, recruit additional native-conformation proteins and polypeptides into amyloidogenic intermediates and that additionally aggregate to form higher-order structures, such as plaques observed in the brains of patients suffering from the various spongiform encephalopathies.
Owner:UNIV OF WASHINGTON
Computationally designed inhibitors of amyloidosis
Embodiments of the present invention include methods and systems for designing inhibitors of amyloidosis in humans, domesticated animals, and wild animals as well as inhibitors of amyloidosis designed by the methods and systems. Methods and systems for designing inhibitors of amyloidosis are largely computational, in nature, and are directed to designing various types of polymers, small-molecule organic compounds, organometallic compounds, or non-chemical physical processes that can target the extended-α-strand and α-sheet regions of amyloidogenic protein and polypeptide intermediates in order to prevent aggregation of those intermediates into protofibrils and fibrils that, in turn, recruit additional native-conformation proteins and polypeptides into amyloidogenic intermediates and that additionally aggregate to form higher-order structures, such as plaques observed in the brains of patients suffering from the various spongiform encephalopathies.
Owner:UNIV OF WASHINGTON
Nucleic acid preparation from whole blood for use in diagnosis of transmissible spongiform encephalopathy
The present invention deals with a method of preparing nucleic acids, particularly RNA, from a whole blood sample. The nucleic acids purified by the method of the invention are particularly suited for detection of nucleic acid marker molecules. Preferred are markers for the diagnosis of transmissible spongiform encephalopathy (TSE). Such diagnosis is based on the detection, by means of real-time PCR, of certain mRNAs of the TSE-infected organism. Said mRNAs specifically originate as splicing variants and are isolated from whole blood by the method of the invention.
Owner:ROCHE DIAGNOSTICS OPERATIONS INC
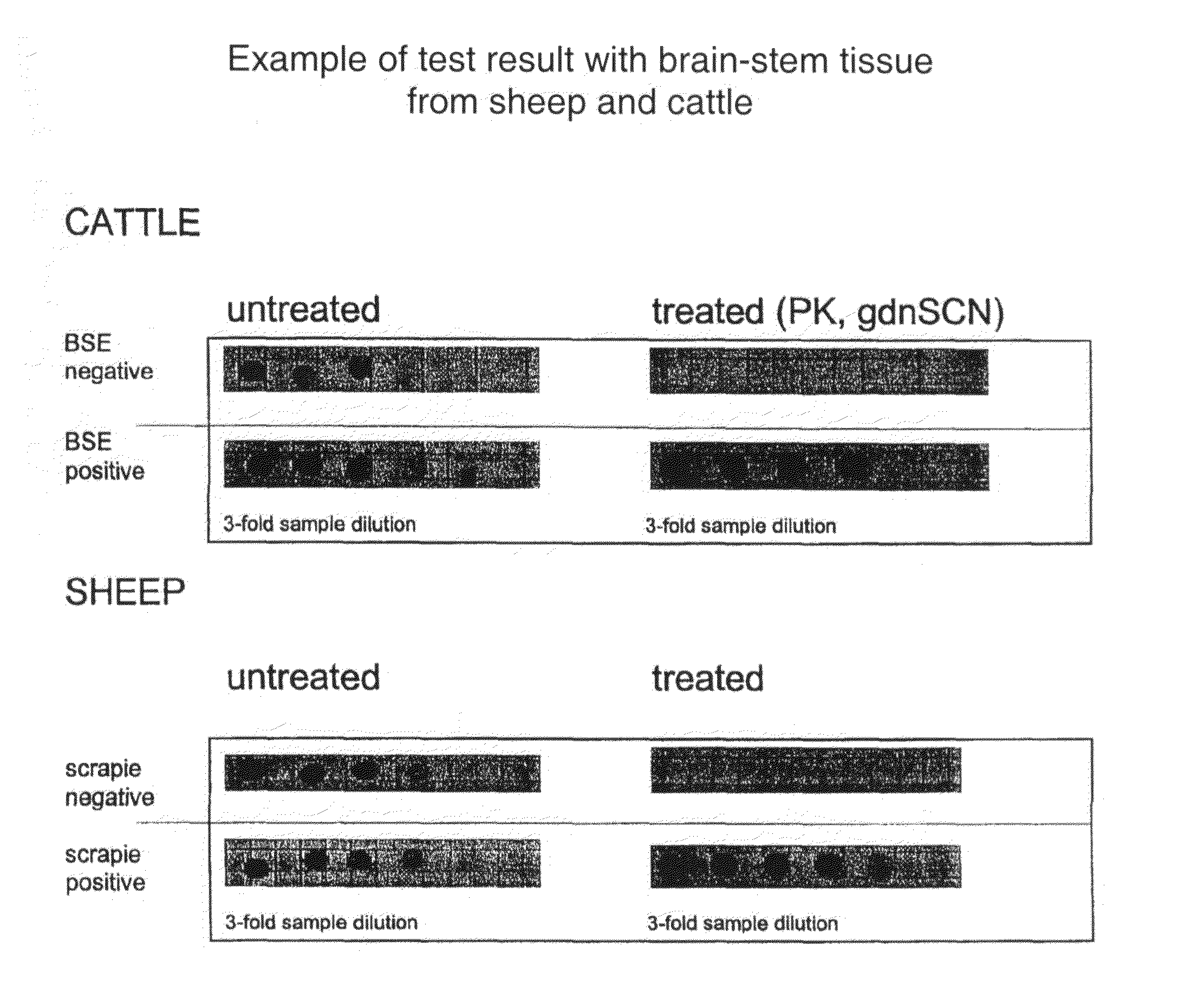
Prion test
InactiveUS20080153117A1Reduce riskReduce signalingPeptide/protein ingredientsMicrobiological testing/measurementGuanidine thiocyanateMammal
The invention is related to diagnostic methods for detecting transmissible spongiform encephalopathies (TSEs) such as BSE and scrapie and related disease in humans. The invention provides use of guanidine thiocyanate (gdnSCN), or a functional equivalent thereof, for treating at least one sample derived from a mammal, including humans, for reducing the risk of scoring a false-positive test result in testing the sample for the presence or absence of aberrant prion protein.
Owner:ID LELYSTAD INSTITUUT VOOR DIERHOUDERIJ EN DIERGEZONDHEID BV
Method for preparing a prion-free bond grafting substitute
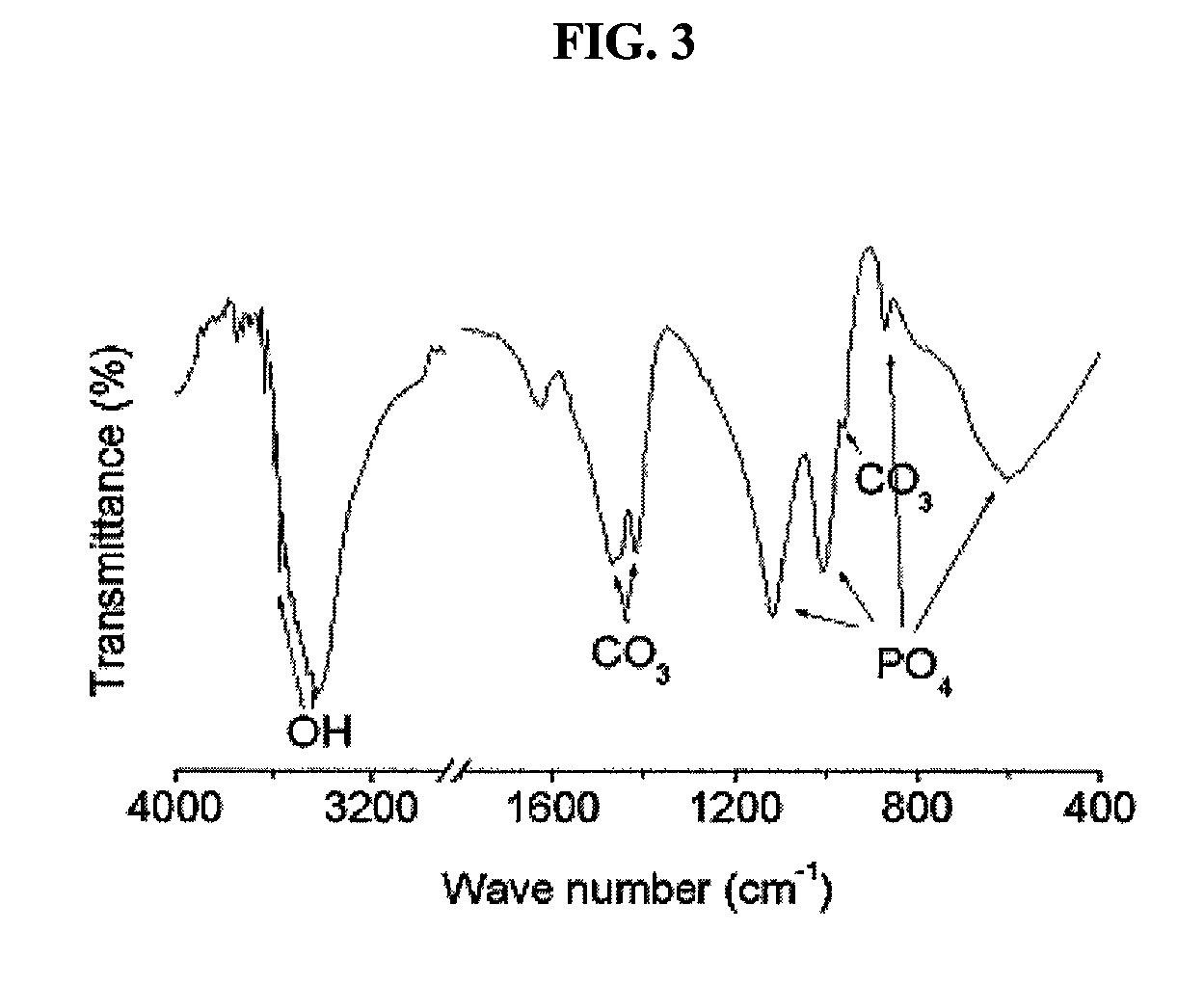
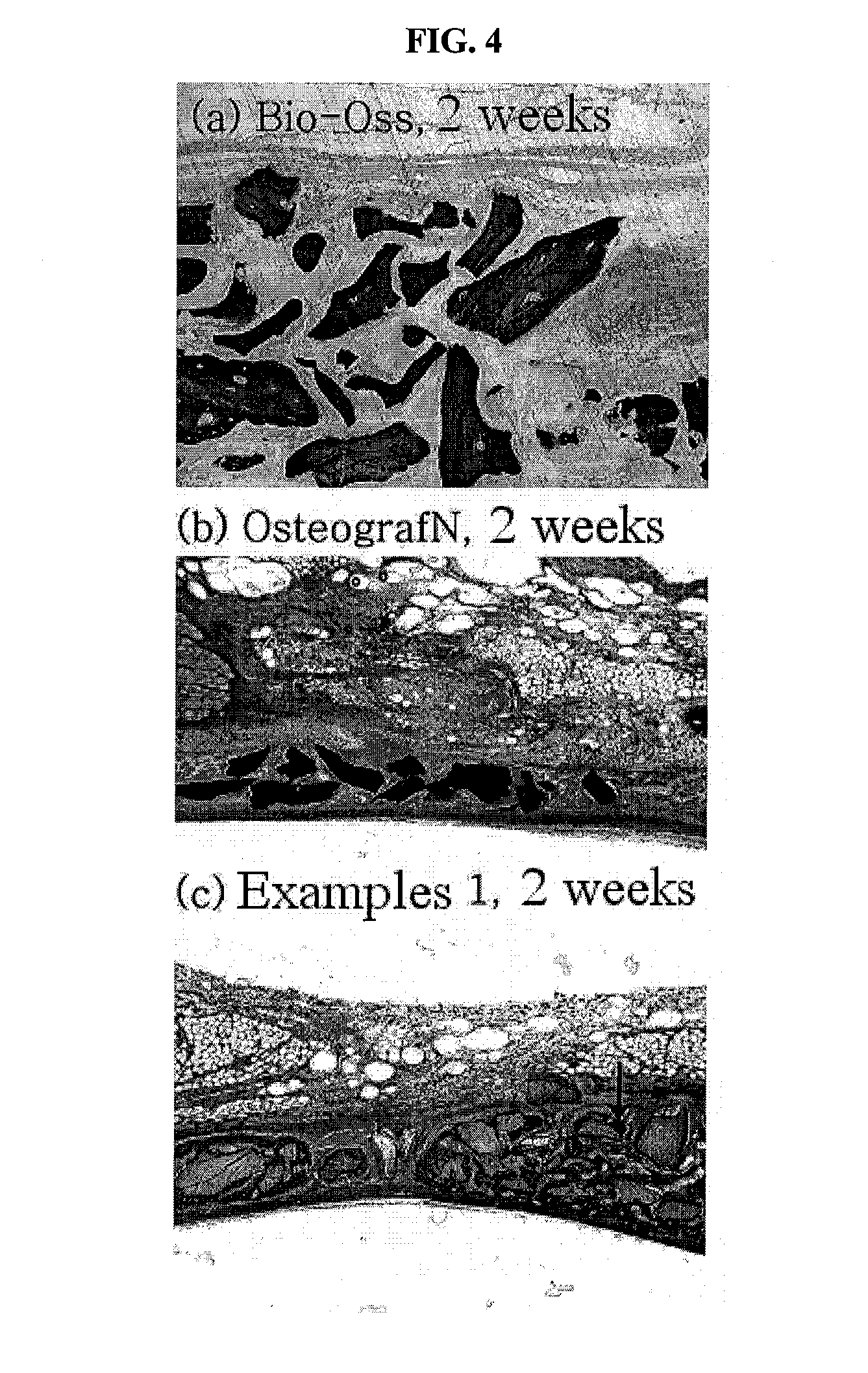
The present invention relates to a method for preparing a bone graft substitute using bovine bone, and more particularly to a method for preparing a safe bone graft substitute which does not have the risk of infection with bovine spongiform encephalopathy, the method comprising treating bovine bone with sodium hypochlorite and treating the treated bone at a high temperature of more than 600° C. The bone graft substitute does not cause an immune response, because it is prepared by effectively removing lipids and organic substances from bovine bone having a structure very similar to that of the human bone. Also, it has excellent osteoconductivity, and is free of prion, and thus it does not have the risk of infection with bovine spongiform encephalopathy. According to the disclosed invention, the bone graft substitute having such advantages can be prepared in a simple manner.
Owner:SEOUL NAT UNIV R&DB FOUND
Mutant proteins and use thereof for the manufacture of medicaments and the treatment of humans or animals suffering from conformational diseases
InactiveUS20050244806A1Improve compatibilityNervous disorderPeptide/protein ingredientsTherapeutic treatmentSomatic cell
The invention relates to a mutant prion protein (PrP), the globular domain of which comprises an engineered second disulfide bond in a similar position as in the human doppel protein (hDpl). In an embodiment, the prion protein has an engineered extra disulfide bond in the presumed ‘factor X’ binding epitope and is accommodated with slight, strictly localized conformational changes to inhibit prion propagation in human and animals. Also disclosed is the use of a mutant prion protein (PrP), the globular domain of which comprises at least one engineered additional disulfide bond in a similar position as in the human doppel protein, or fragments thereof for therapeutic treatment or for the manufacture of a medicament for therapeutic treatment of proteins causing disease after a conformational transition, e.g. Tranmissible Spongiform Encephalopathy (TSE), variant forms of Creutzfeldt-Jakob disease (CJD), fatal familial insomnia (FFI), and Gerstmann-Sträussler-Scheinker syndrome (GSS) in human. Further, the use of the PrP mutant protein for in vivo generation of disulfide mutants of prion proteins or fragments thereof is carried out in order to enable an intended ther-apy of TSE in animals, e.g. by somatic gene therapy with lentiviral vector, where TSE includes bovine spongiform encephalopathy (BSE), scrapie in sheep, feline spongiform encephalopathy (FSE), and chronic wasting disease (CWD) in elk and deer.
Owner:ETH ZZURICH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com