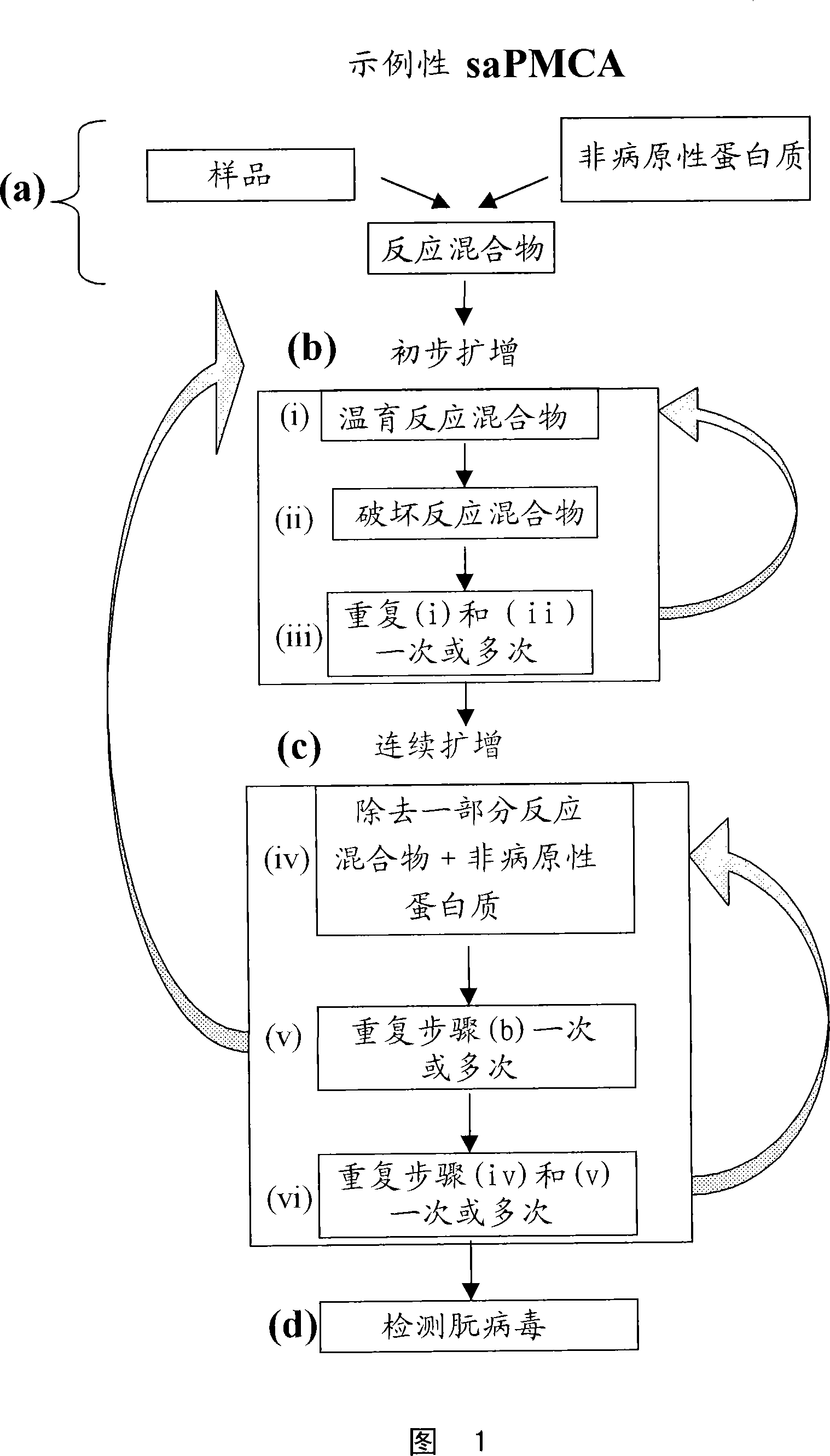
Ultrasensitive detection of prions by automated protein misfolding cyclic amplification
A prion, protein technology, applied in 0004] The present invention generally relates to pathology, a method, a composition and a device for disease, enjoying the present invention, detecting protein or prion in a sample, biochemistry and cell biology. field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
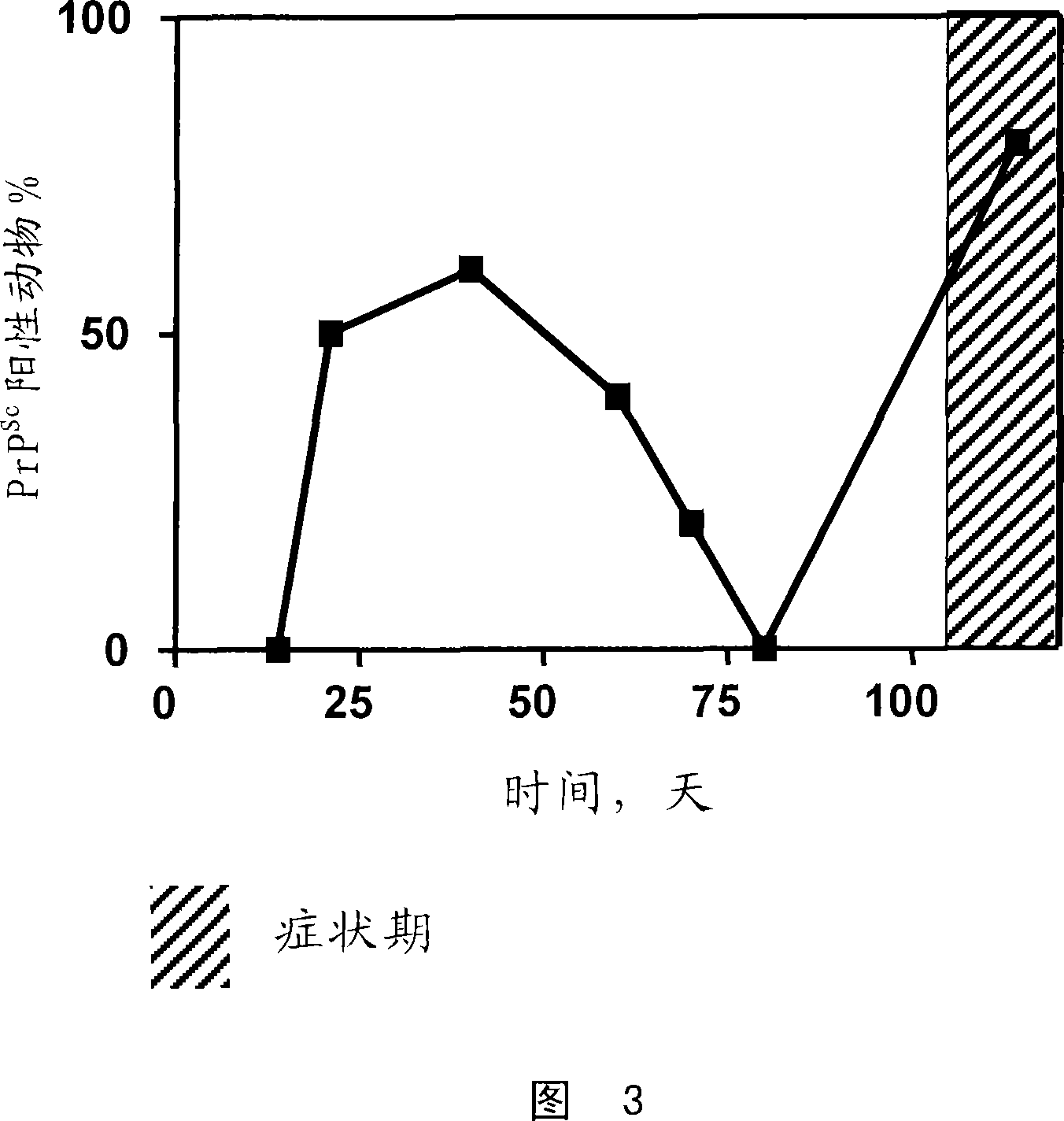
Image
Examples
Embodiment 1
[0170] Purification of PrP from brain Sc
[0171] 1 g of brain tissue was homogenized in 5 ml cold PBS containing protease inhibitors. For PrP generated by PMCA res, after final purification, the gross sample containing normal brain homogenate used as substrate was processed in the same manner as the brain homogenate. Samples were mixed with 1 volume of 20% sarcosyl and the mixture was homogenized and sonicated until a clear preparation was obtained. Samples were centrifuged at 5000 rpm for 15 minutes at 4°C. Discard the pellet and mix the supernatant with 1 / 3 volume of PBS containing 0.1% SB-314 and centrifuge at 100,000 x g for 3 h at 4 °C in a Biosafe Optima MAX ultracentrifuge (Beckman Coulter, Fullerton, CA) . Discard the supernatant and resuspend the pellet in 600 μl of PBS containing 0.1% SB-314, 10% NaCl and sonicate. The resuspended pellet was layered on 600 ul of PBS containing 20% sucrose, 10% NaCl and 0.1% SB 3-14 and centrifuged at 4°C f...
Embodiment 2
[0173] Automation: Increase throughput and reduce amplification time
[0174] The use of single-probe conventional sonicators poses practical problems for processing many samples simultaneously, as would be required for diagnostic testing. The present invention has transformed the cycle amplification system into a 96-well microplate sonicator (Misonix TM Model 3000 (Farmingdale, N.Y.)), which provides sonication to all wells simultaneously and can be programmed for automatic operation. This improvement not only reduces processing time and increases throughput, but also allows routinely more cycles than single-probe sonicators. Cross-contamination is eliminated because the probe does not penetrate the sample directly. This is important for handling infectious samples and reducing false positive results. 10 cycles of 1 hour incubation followed by 30 s sonication pulses yielded PrP Sc Significant amplification of the signal, similar to that observed with conventi...
Embodiment 3
[0176] Increased amplification efficiency through metal chelation
[0177] As part of our efforts to optimize the PMCA procedure, the inventors discovered that the relatively low levels of amplification previously observed were due in part to the presence of metal cations in the samples. The efficiency of amplification was dramatically higher when the PMCA reaction was performed in the presence of EDTA, a broad-range metal chelator.
[0178] It is well established that PrP binds copper with high affinity (and zinc to a lesser degree) and that a likely biological function of the virtually normal prion protein would be to involve Cu 2+ transport across the cell membrane (Brown and Sassoon, 2002). The results show that when Cu is added to the reactant 2+ , the positive role of EDTA in enhancing the efficiency of PMCA was lost. This effect is very clear and concentration dependent. with other divalent cations such as Ca 2+ and Mg 2+ No significant effect was...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com