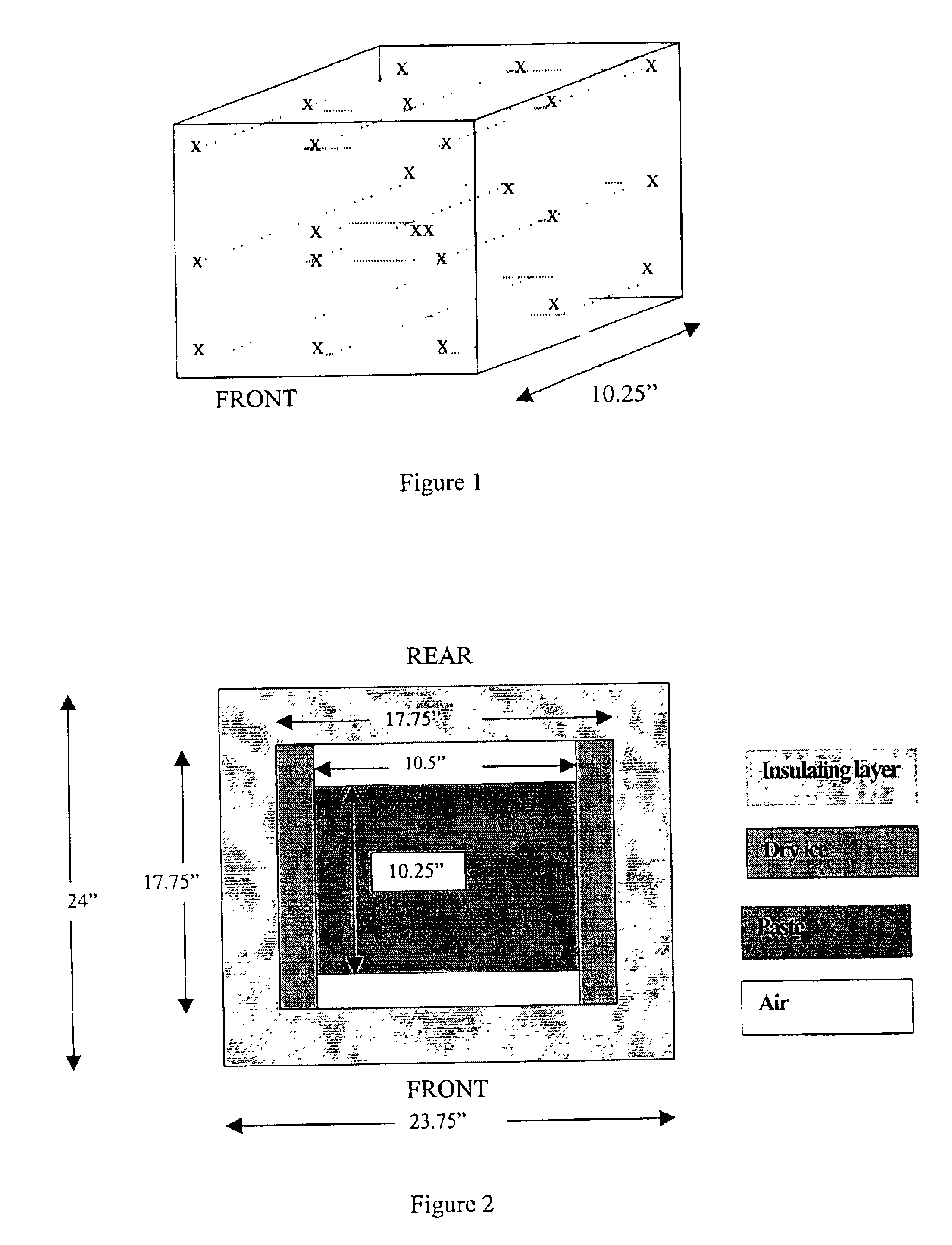
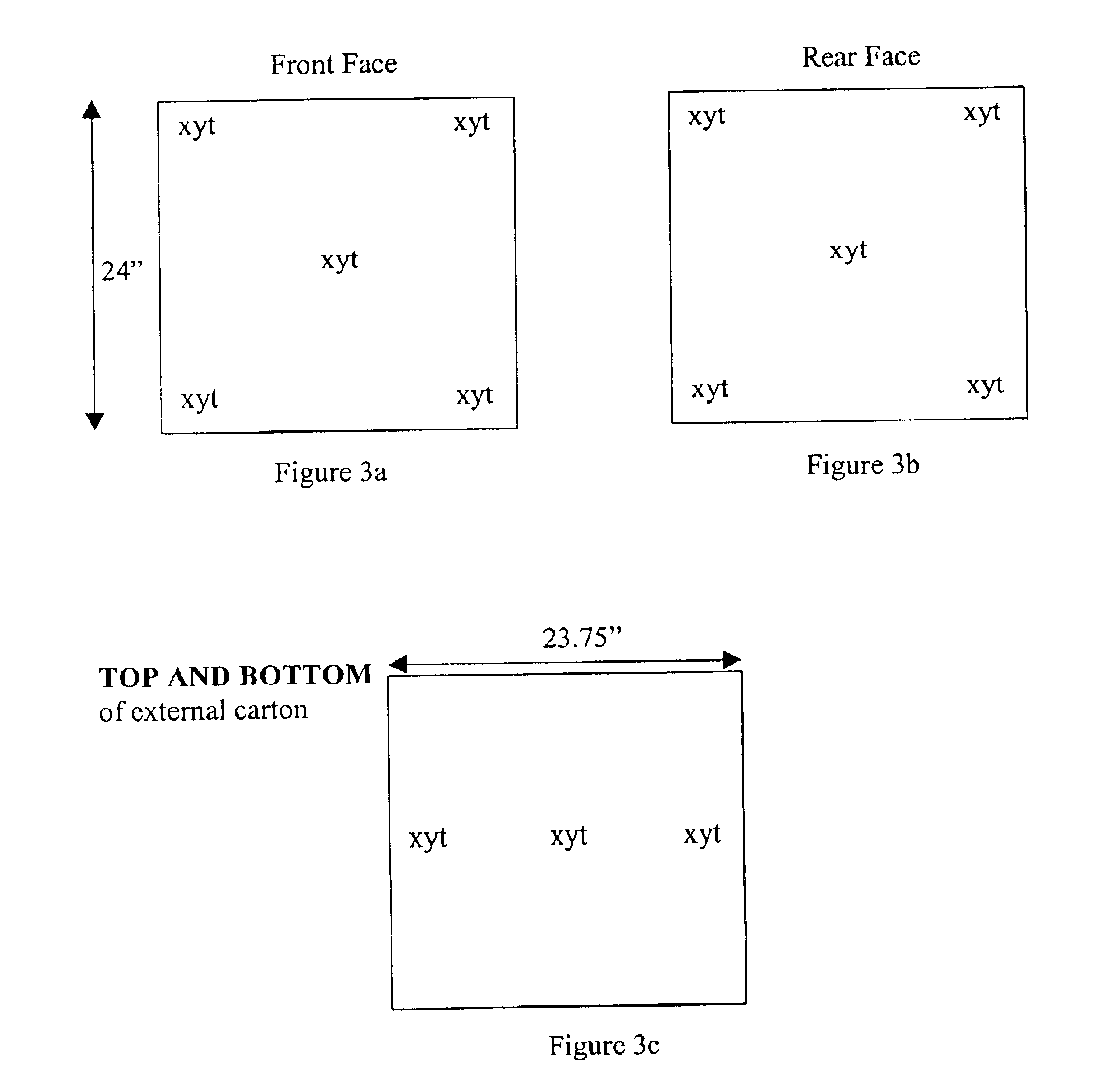
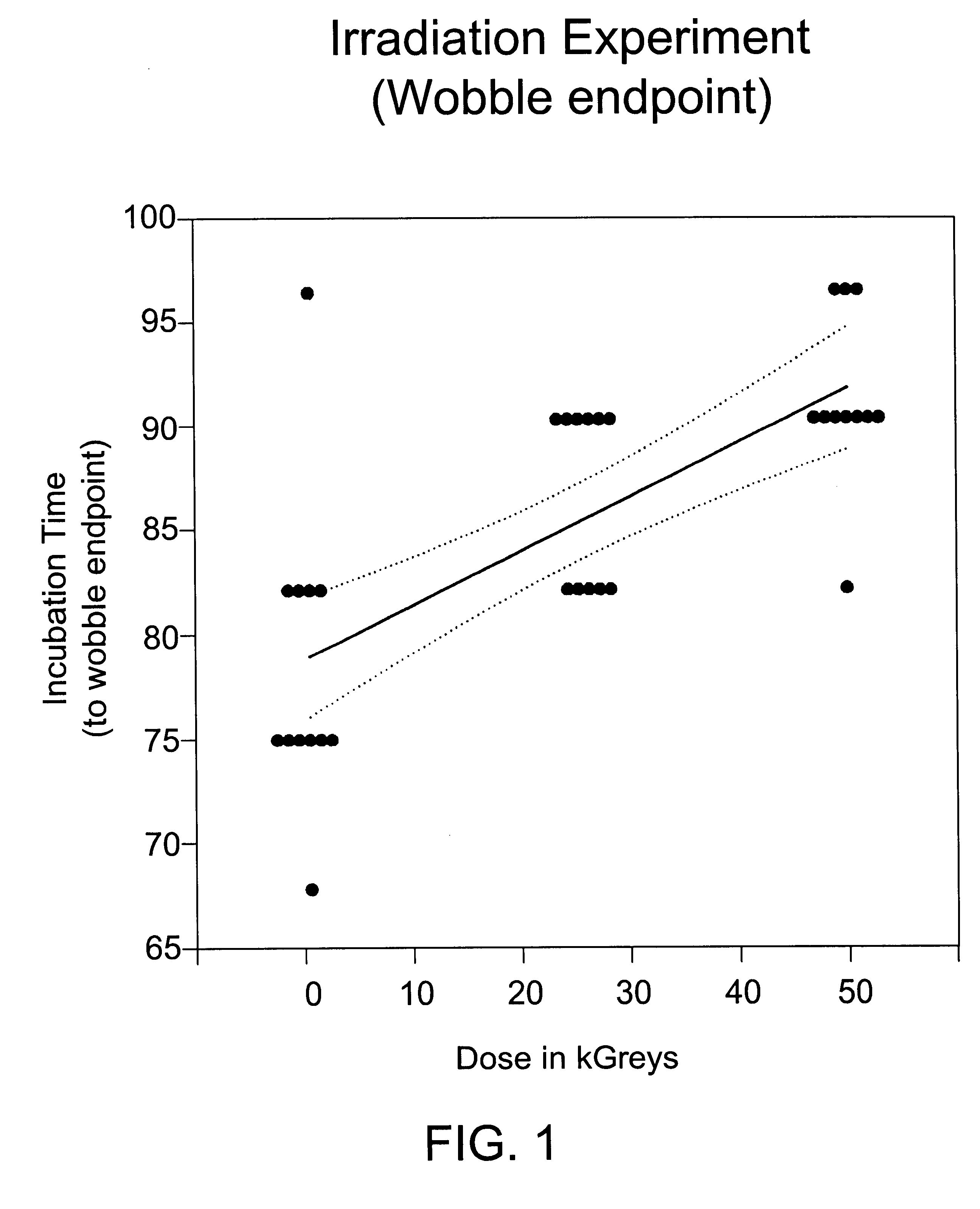
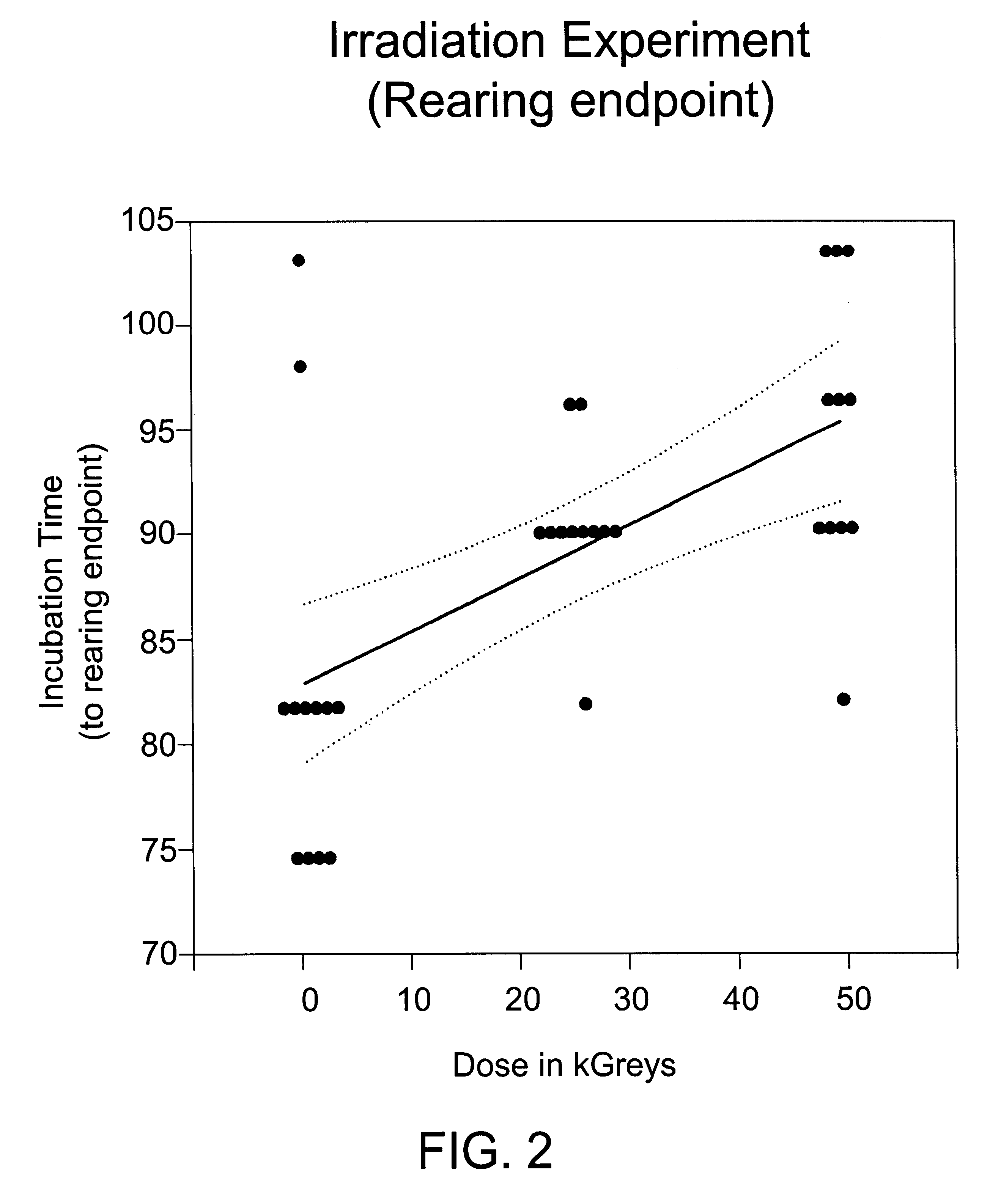
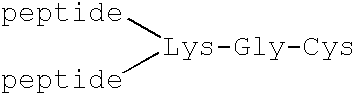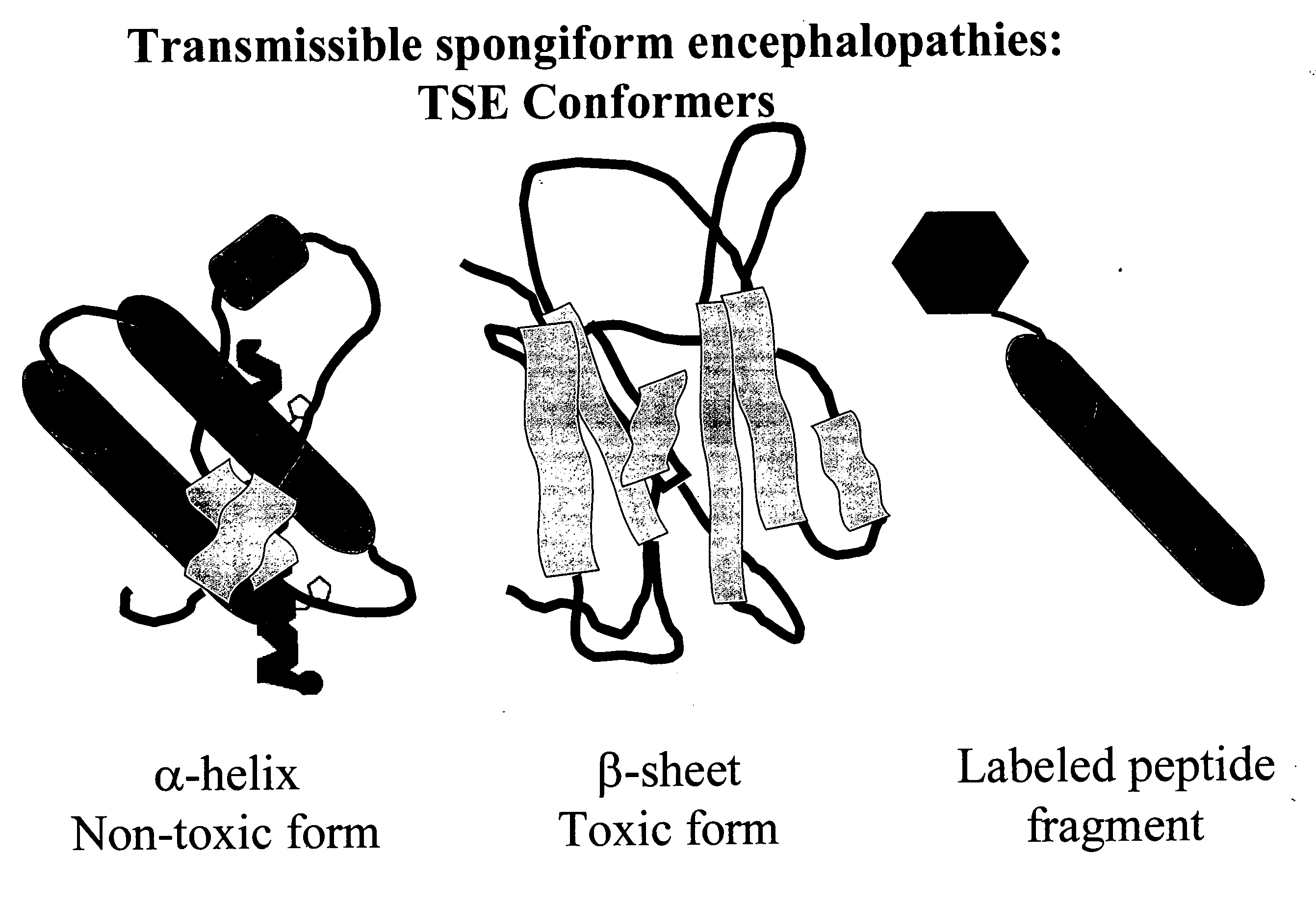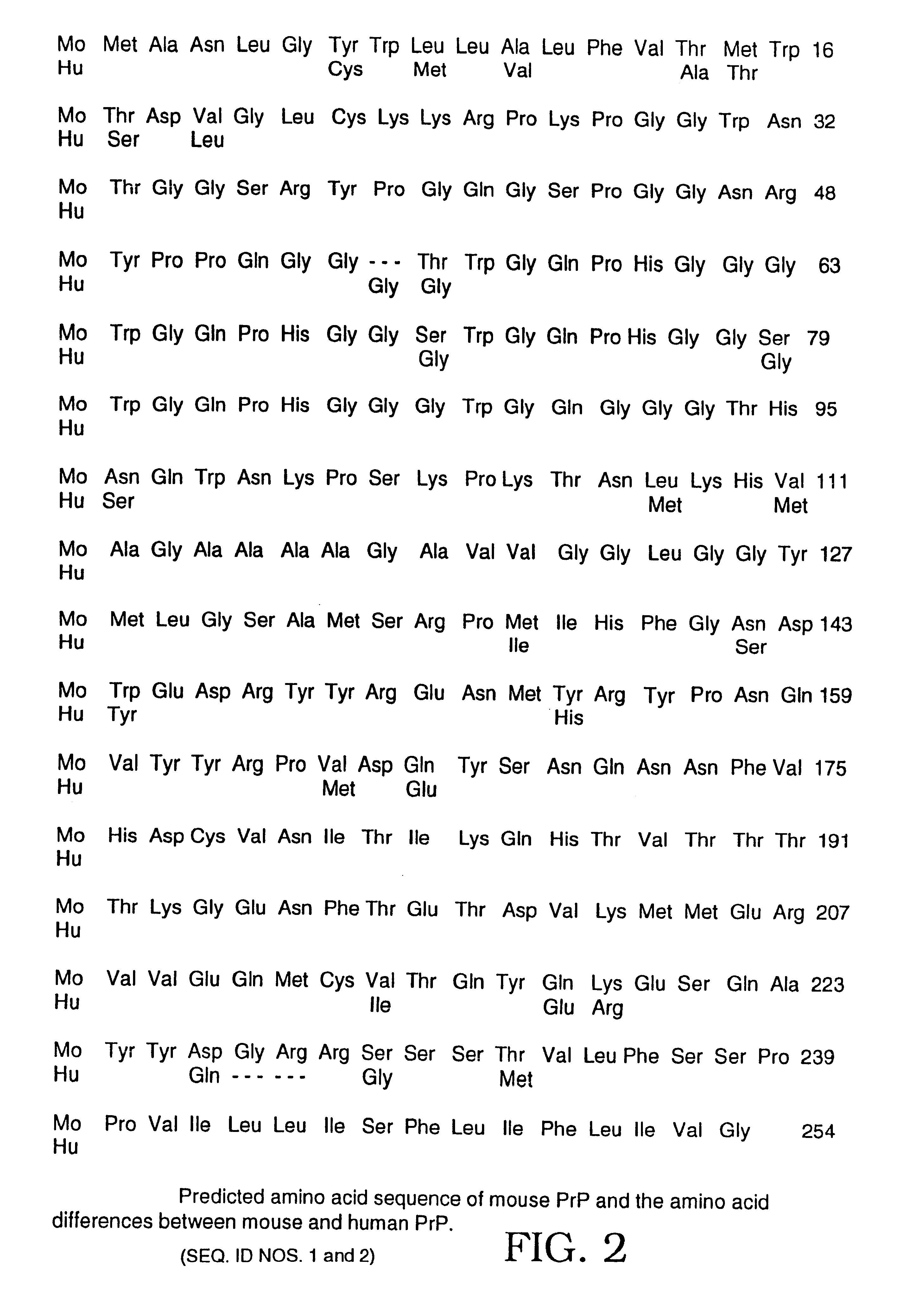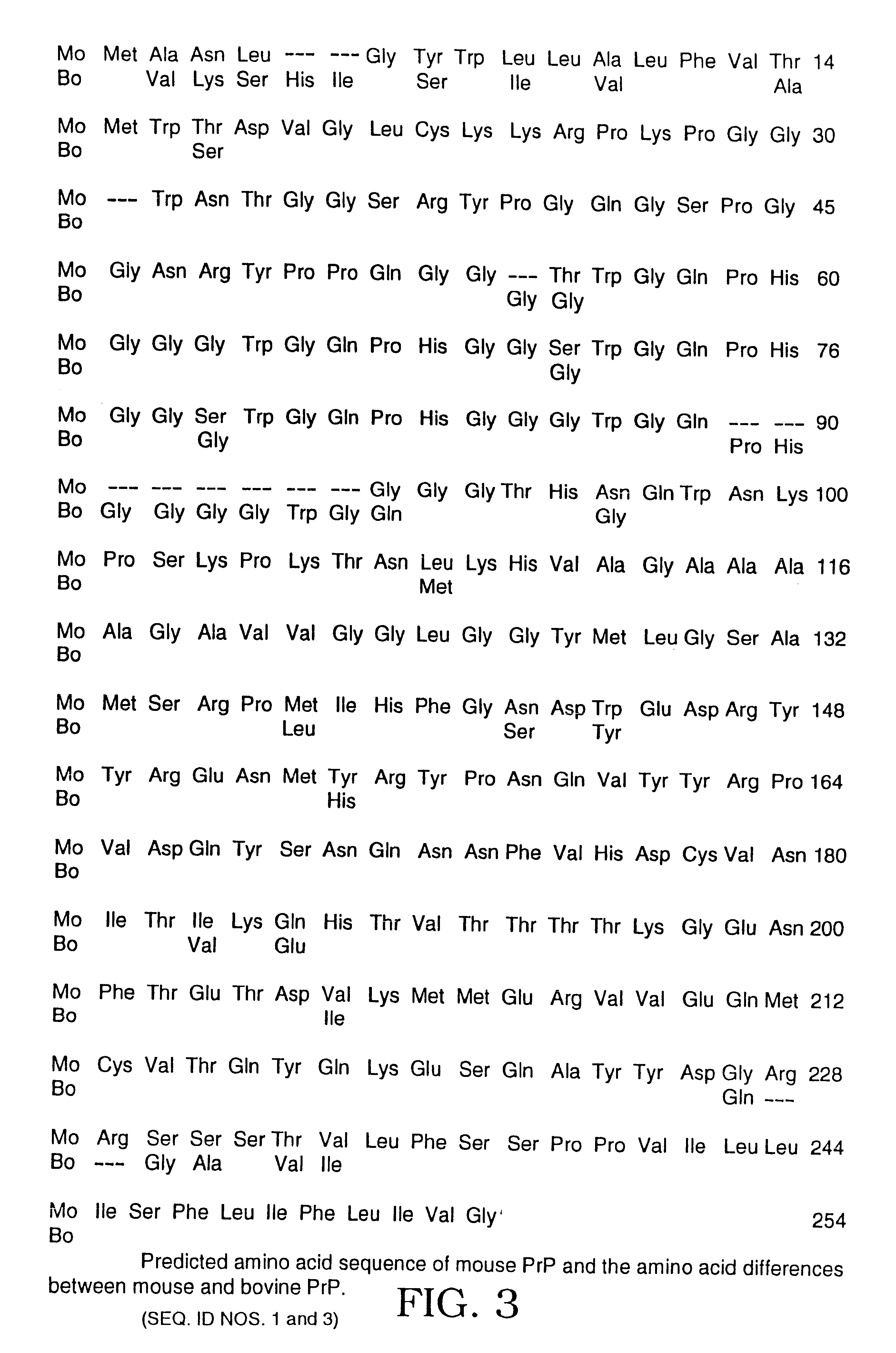Patents
Literature
255 results about "Proteinaceous infectious particle" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
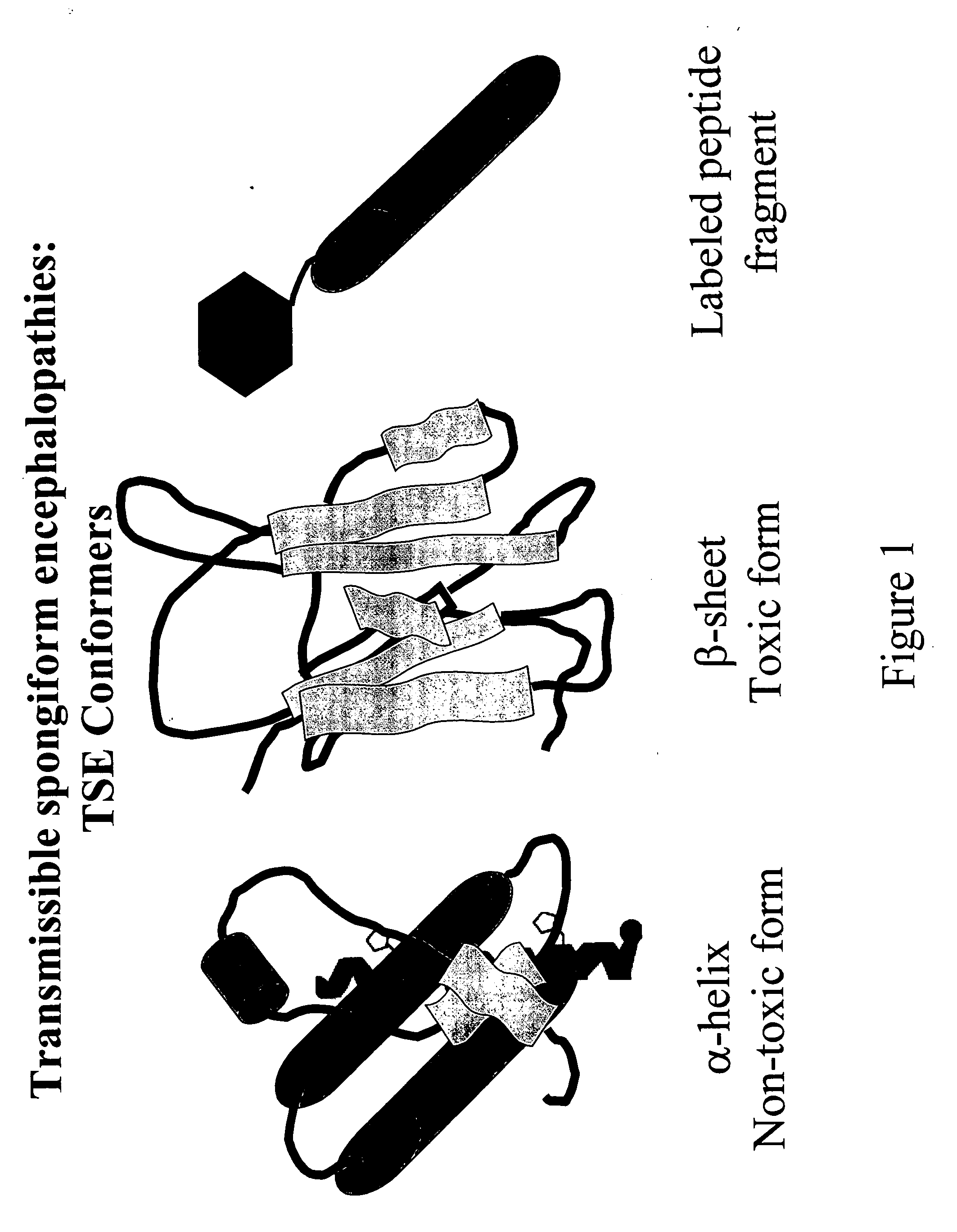
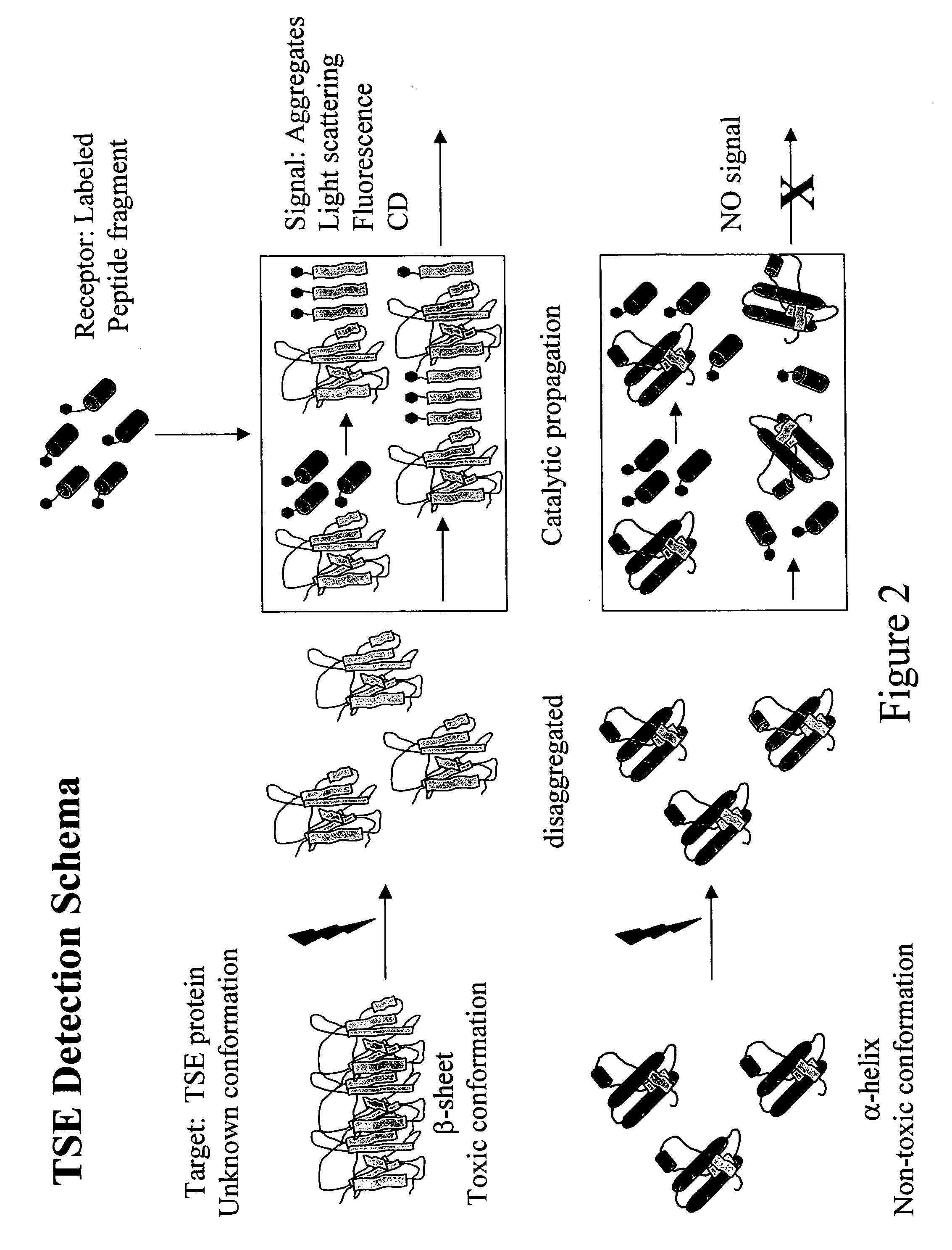
Prions are misfolded proteins with the ability to transmit their misfolded shape onto normal variants of the same protein. They characterize several fatal and transmissible neurodegenerative diseases in humans and many other animals. It is not known what causes the normal protein to misfold, but the abnormal three-dimensional structure is suspected of conferring infectious properties, collapsing nearby protein molecules into the same shape. The word prion derives from "proteinaceous infectious particle". The hypothesized role of a protein as an infectious agent stands in contrast to all other known infectious agents such as viruses, bacteria, fungi and parasites, all of which contain nucleic acids (DNA, RNA or both).
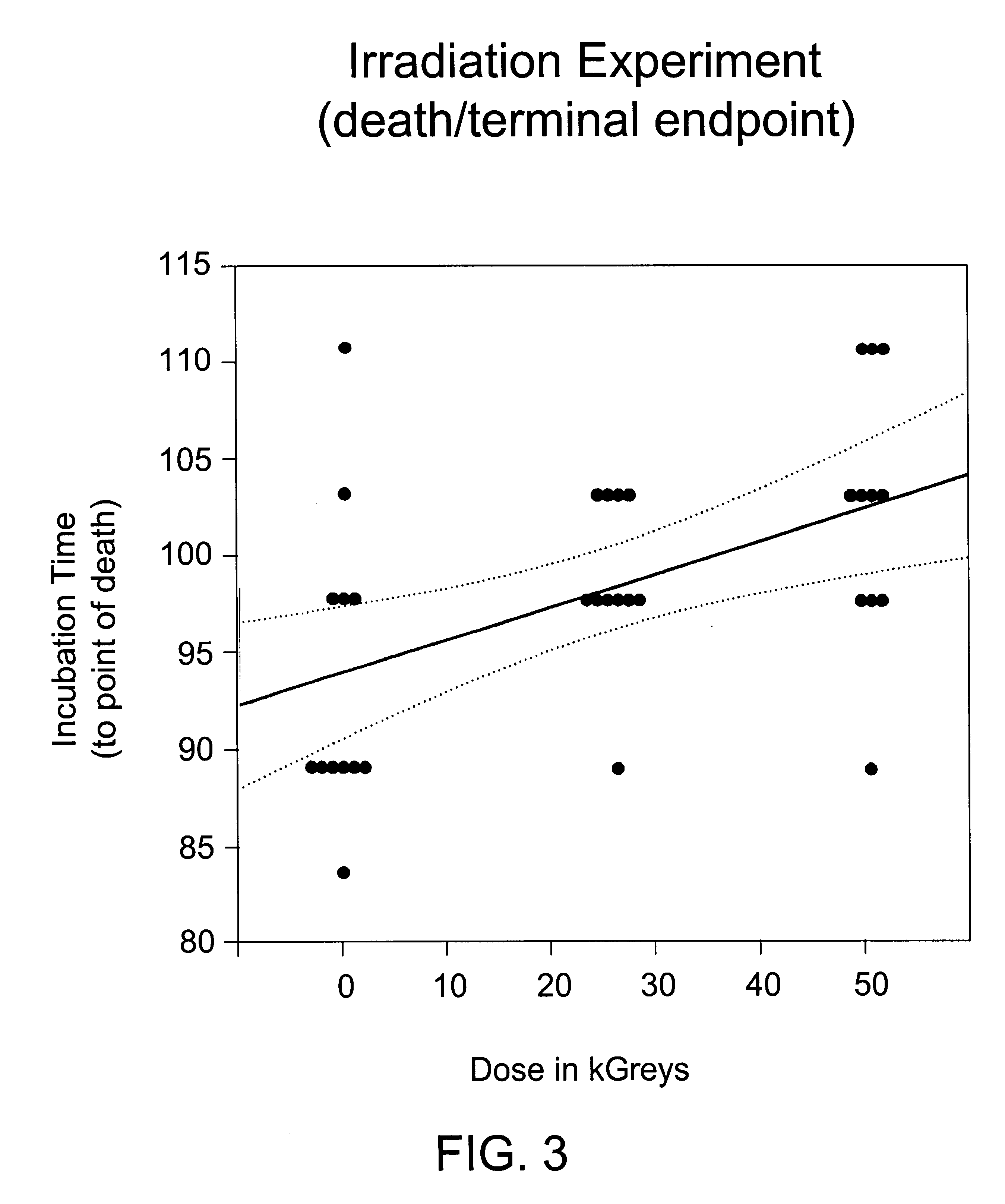
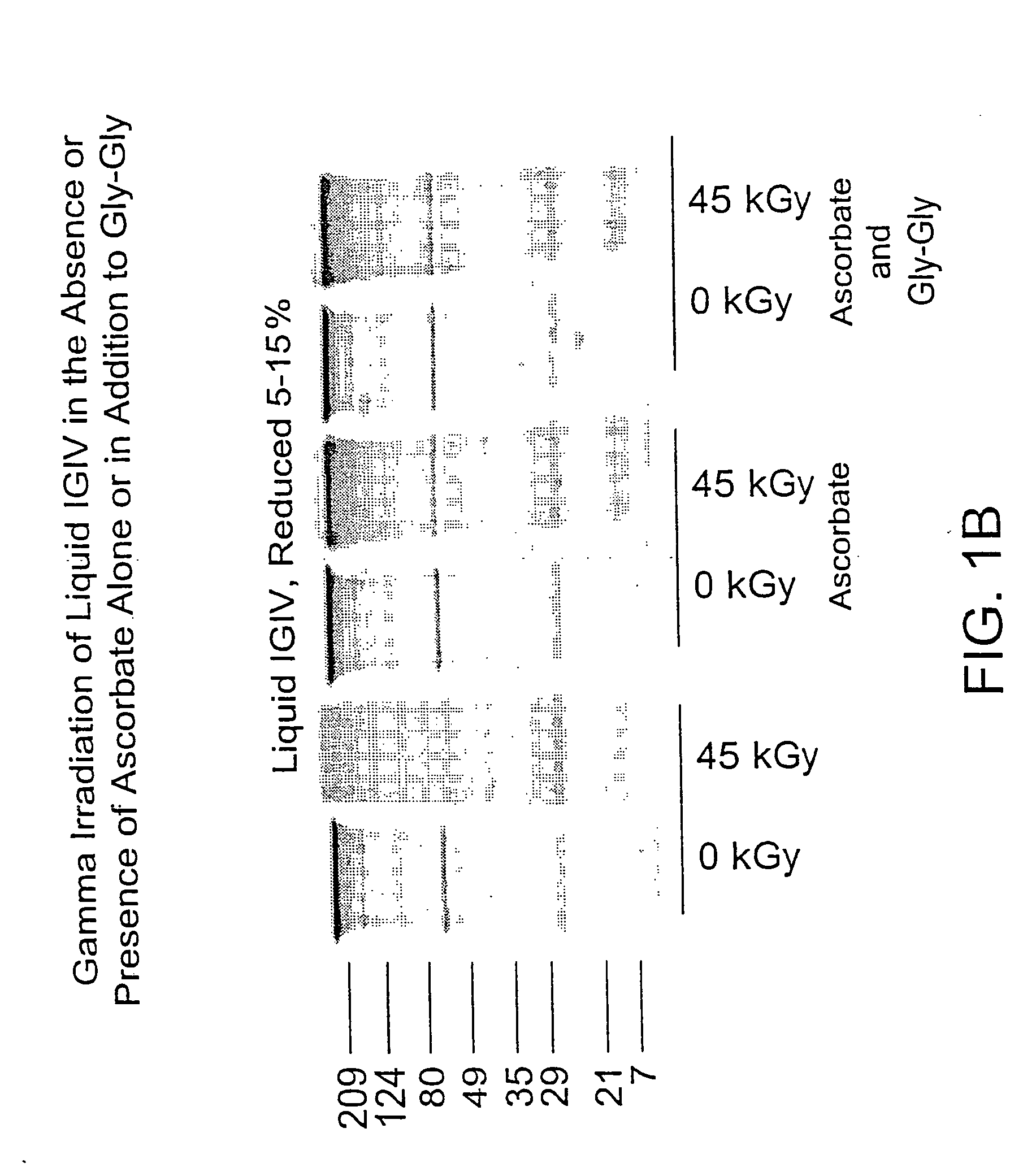
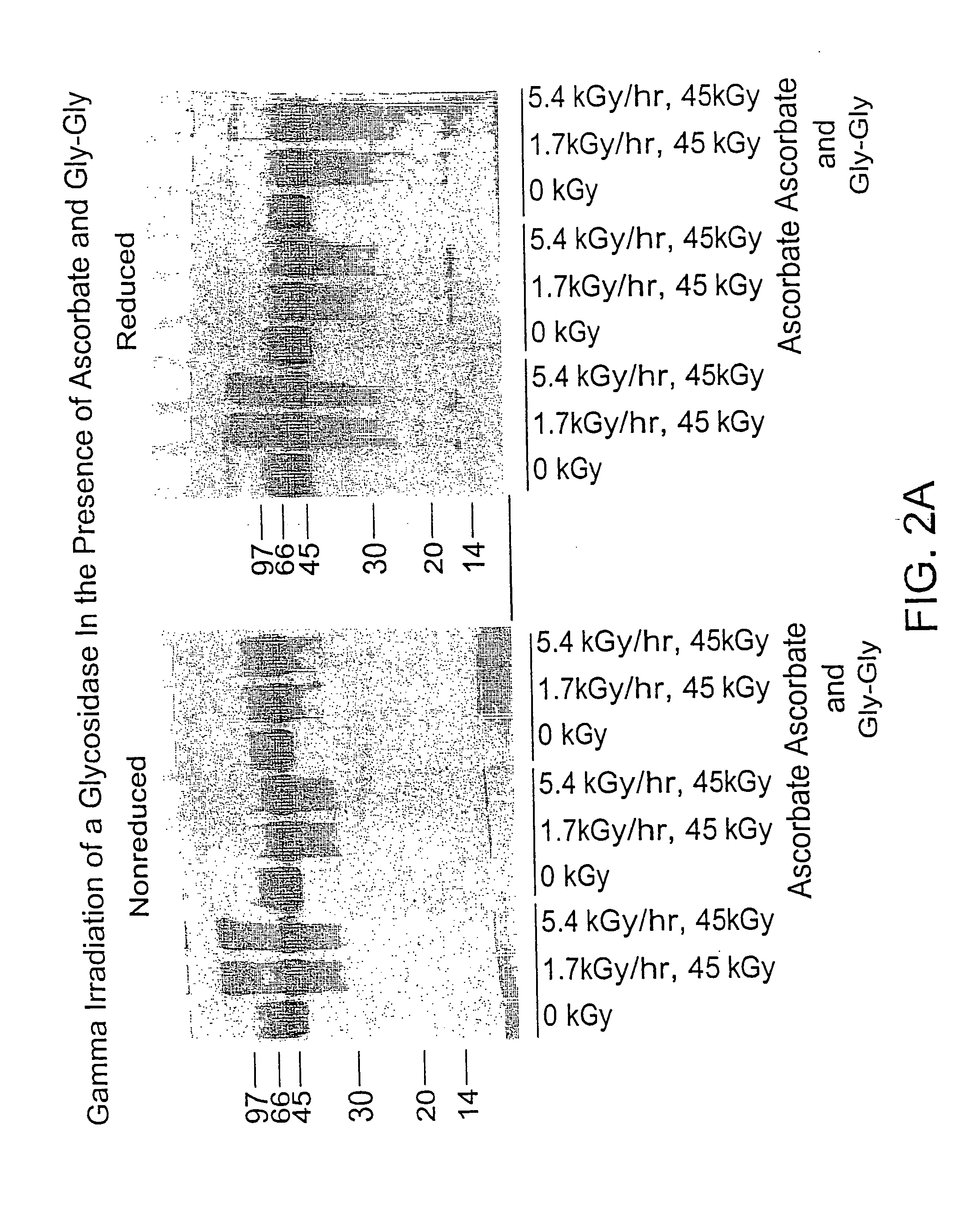
Methods for sterilizing biological materials by irradiation over a temperature gradient
InactiveUS6908591B2Effective sterilizationImprove permeabilityDead animal preservationLavatory sanitoryBiological materialsBiology
Methods are disclosed for sterilizing tissue to reduce the level of one or more active biological contaminants or pathogens therein, such as viruses, bacteria, (including inter- and intracellular bacteria, such as mycoplasmas, ureaplasmas, nanobacteria, chlamydia, rickettsias), yeasts, molds, fungi, spores, prions or similar agents responsible, alone or in combination, for TSEs and / or single or multicellular parasites. The methods involve sterilizing one or more tissues with irradiation.
Owner:CLEARANT
Methods for sterilizing biological materials
InactiveUS6946098B2Lowered residual solventReduce the temperatureOther blood circulation devicesFood preservationBiochemistryBiological materials
Methods are disclosed for sterilizing biological materials to reduce the level therein of one or more biological contaminants or pathogens, such as prions, responsible for the disease states known as transmissible spongiform encephalopathies (TSEs) in mammals. These methods involve sterilizing biological materials with irradiation.
Owner:CLEARANT
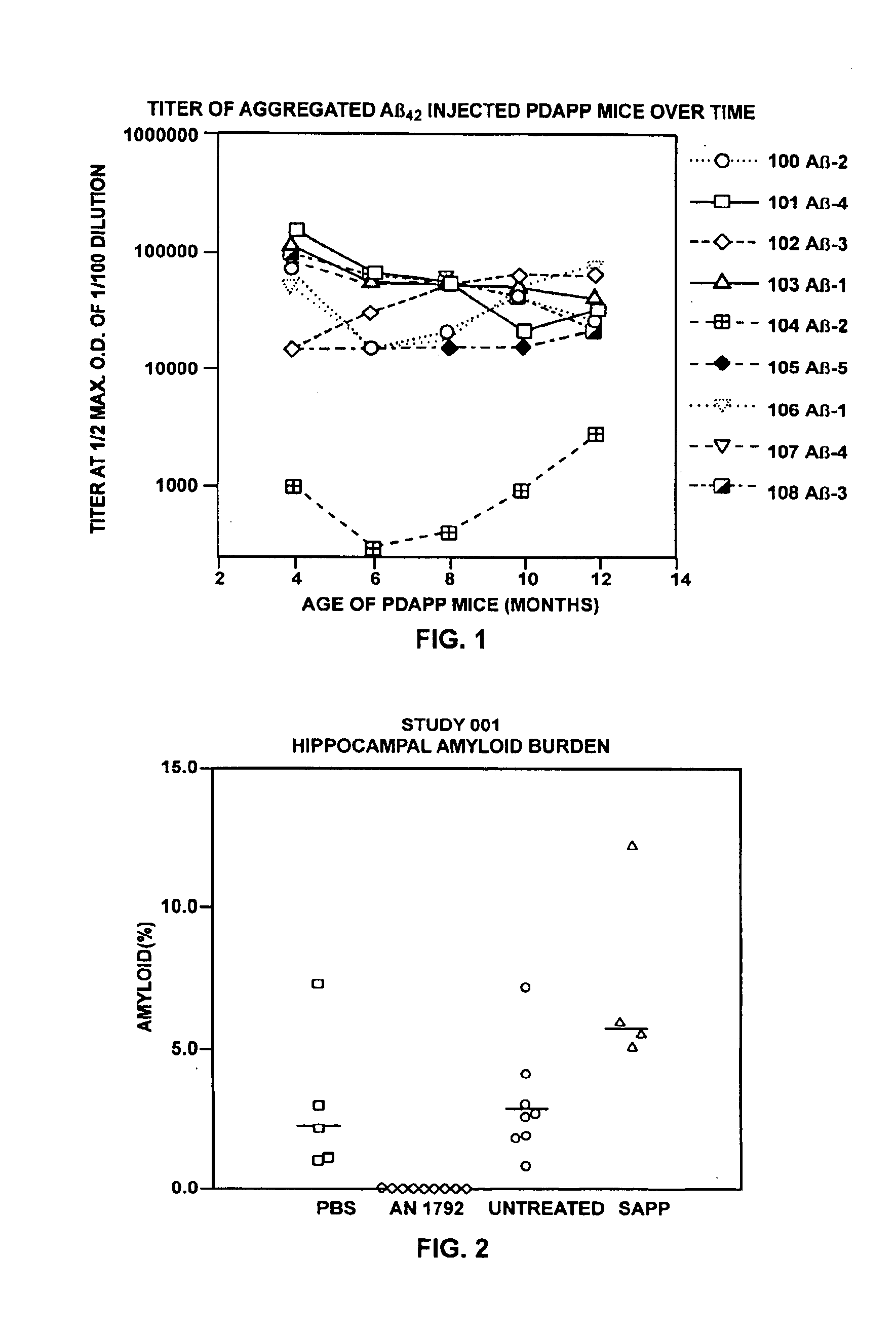
Passive immunization of Alzheimer's disease
InactiveUS6913745B1Peptide/protein ingredientsAntibody mimetics/scaffoldsPassive ImmunizationsAmyloid disease
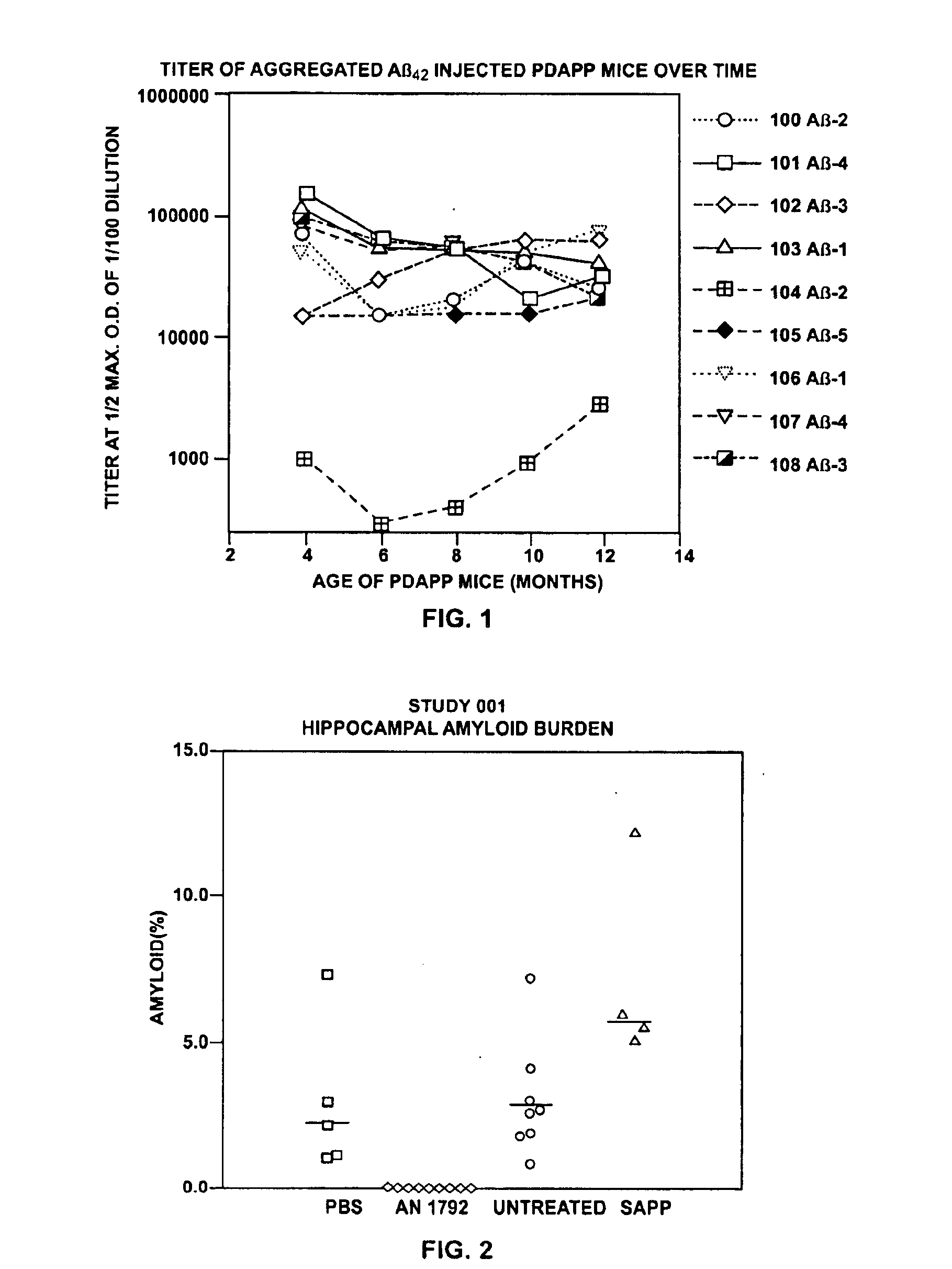
Disclosed are pharmaceutical compositions and methods for preventing or treating a number of amyloid diseases, including Alzheimer's disease, prion diseases, familial amyloid neuropathies and the like. The pharmaceutical compositions include immunologically reactive amounts of amyloid fibril components, particularly fibril-forming peptides or proteins. Also disclosed are therapeutic compositions and methods which use immune reagents that react with such fibril components.
Owner:JANSSEN SCI IRELAND UC
Pharmaceutical compositions and methods for treatment of amyloid diseases
Disclosed are pharmaceutical compositions and methods for preventing or treating a number of amyloid diseases, including Alzheimer's disesase, prion diseases, familial amyloid neuropathies and the like. The pharmaceutical compositions include immunologically reactive amounts of amyloid fibril components, particularly fibril-forming peptides or proteins. Also disclosed are therapeutic compositions and methods which use immune reagents that react with such fibril components.
Owner:JANSSEN ALZHEIMER IMMUNOTHERAPY
Prion-free collagen and collagen-derived products and implants for multiple biomedical applications; methods of making thereof
InactiveUS6197935B1Preserve integrityPeptide/protein ingredientsImmunoglobulinsIn vivo biocompatibilityCollagen VI
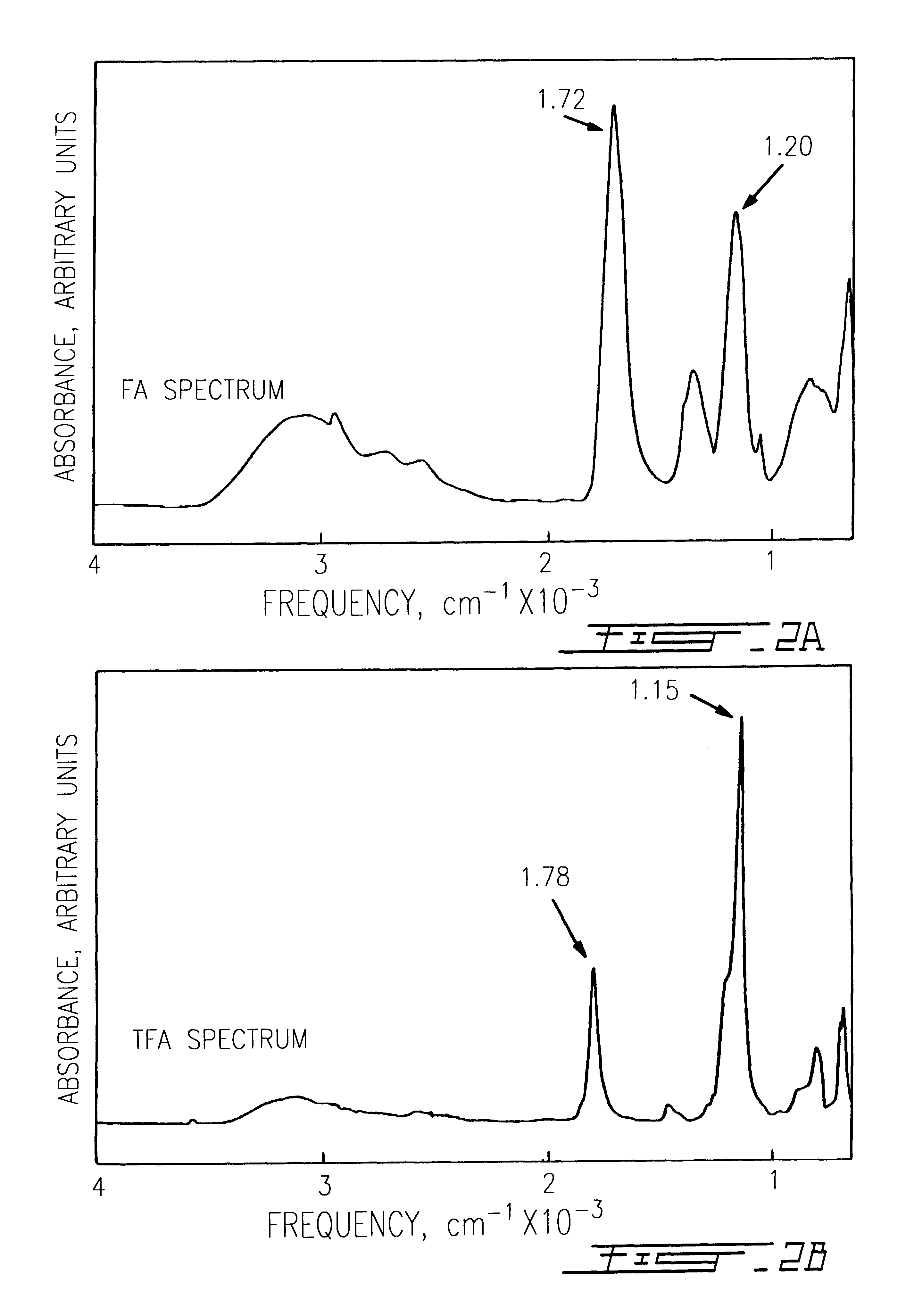
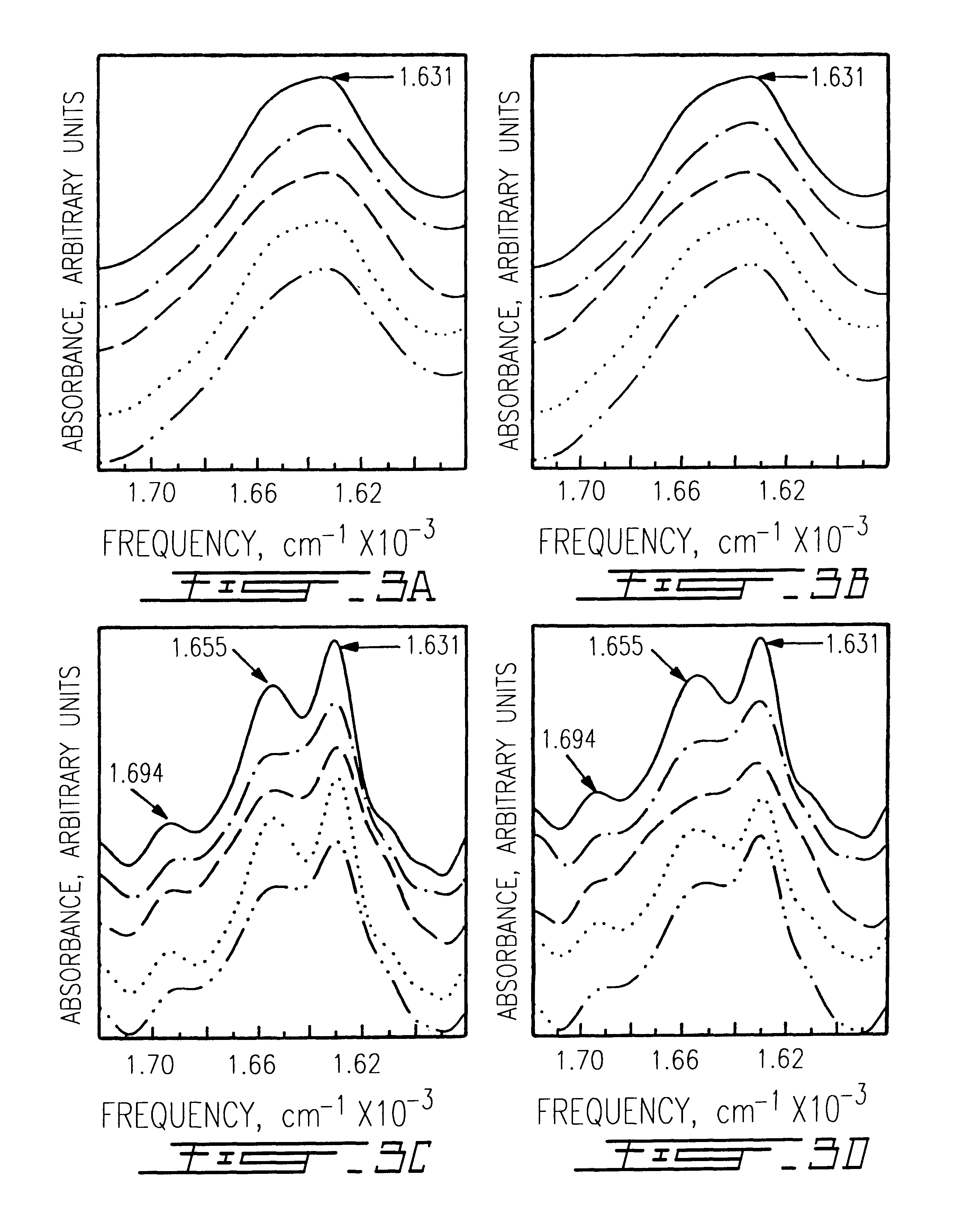
The use of collagen as a biomedical implant raises safety issues towards viruses and prions. The physicochemical changes and the in vitro and in vivo biocompatibility of collagen treated with heat, and by formic acid (FA), trifluoroacetic acid (TFA), tetrafluoroethanol (TFE) and hexafluoroiso-propanol (HFIP) were investigated. FA and TFA resulted in extensive depurination of nucleic acids while HFIP and TFE did so to a lesser degree. The molecules of FA, and most importantly of TFA, remained within collagen. Although these two acids induced modification in the secondary structure of collagen, resistance to collagenase was not affected and, in vitro, cell growth was not impaired. Severe dehydrothermal treatment, for example 110° C. for 1-3 days under high vacuum, also succeeded in removing completely nucleic acids. Since this treatment also leads to slight cross-linking, it could be advantageously used to eliminate prion and to stabilize gelatin products. Finally, prolonged treatment with TFA provides a transparent collagen, which transparency is further enhanced by adding glycosaminoglycans or proteoglycans, particularly hyaluronic acid. All the above treatments could offer a safe and biocompatible collagen-derived material for diverse biomedical uses, by providing a virus or prion-free product.
Owner:UNIV LAVAL
Active immunization of AScr for prion disorders
Disclosed are pharmaceutical compositions and methods for preventing or treating a number of amyloid diseases, including Alzheimer's disease, prion diseases, familial amyloid neuropathies and the like. The pharmaceutical compositions include immunologically reactive amounts of amyloid fibril components, particularly fibril-forming peptides or proteins. Also disclosed are therapeutic compositions and methods which use immune reagents that react with such fibril components.
Owner:PROTHENA BIOSCI LTD
Methods and compositions for the treatment of symptoms of prion diseases
InactiveUS20100092447A1Peptide/protein ingredientsMicrobiological testing/measurementDiseaseFaecal chymotrypsin
A therapeutic composition for the treatment of the symptoms of prion diseases and the method for preparing the therapeutic agents is disclosed. The therapeutic composition is a stable pharmaceutical composition comprising one or more digestive and / or pancreatic enzymes. The therapeutic composition may be manufactured by a variety of encapsulation technologies. Delivery of the therapeutic composition may be made orally, through injection, by adherence of a medicated patch or other method. Further, a method of using fecal chymotrypsin level as a biomarker for the presence of a prion disease, or the likelihood of an individual to develop a prion disease is disclosed.
Owner:CUREMARK
Method for fixing tissue
A method of preparing a fixed biological tissue includes, shaping the biological tissue, cross-linking the biological tissue, sterilizing the biological tissue, and inactivating prions in the biological tissue.
Owner:THE CLEVELAND CLINIC FOUND
Removal of prions from blood, plasma and other liquids
InactiveUS6221614B1High affinityHigh affinity bindingImmobilised enzymesHaemofiltrationBlood plasmaProteinaceous infectious particle
Devices such as flow through columns, substrates such as spherical polymer beads, and methods of using such to remove prions from any liquid sample are disclosed. A surface of a substrate is coated with a prion complexing agent, such as a salt of phosphotungstic acid. Blood or plasma passing through a column containing beads coated with prion complexing agent are rendered prion free.
Owner:RGT UNIV OF CALIFORNIA
Immunological detection of prions
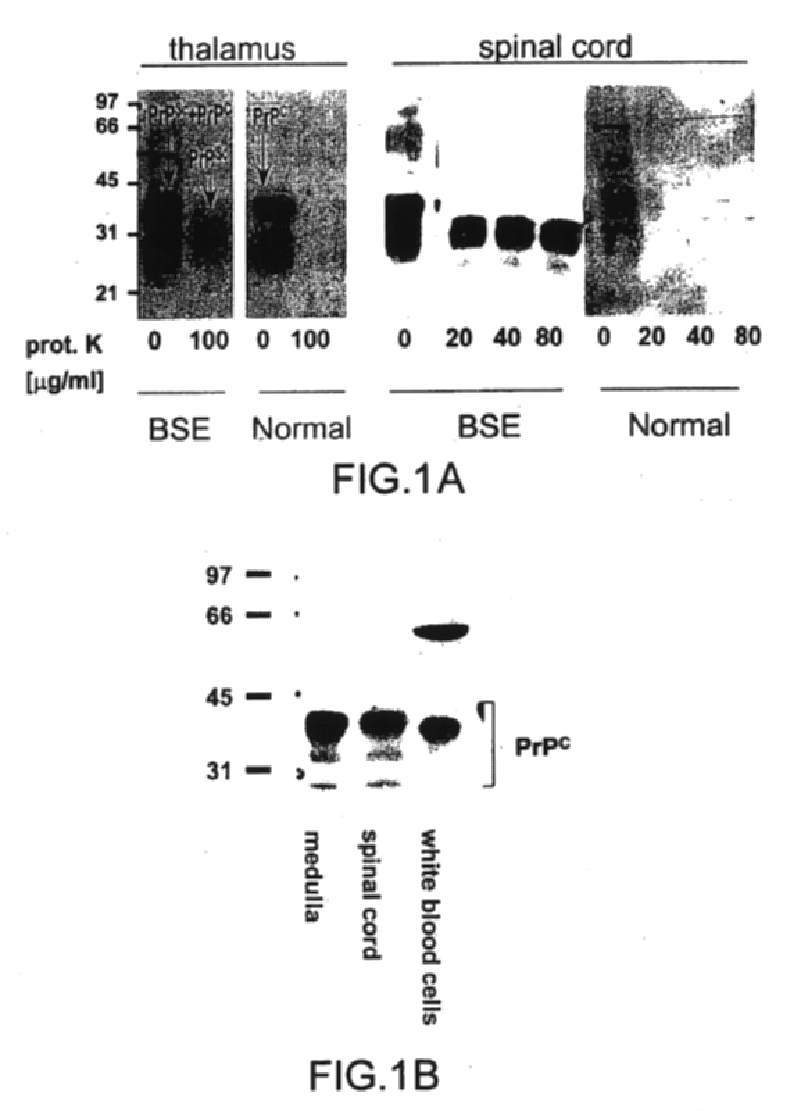
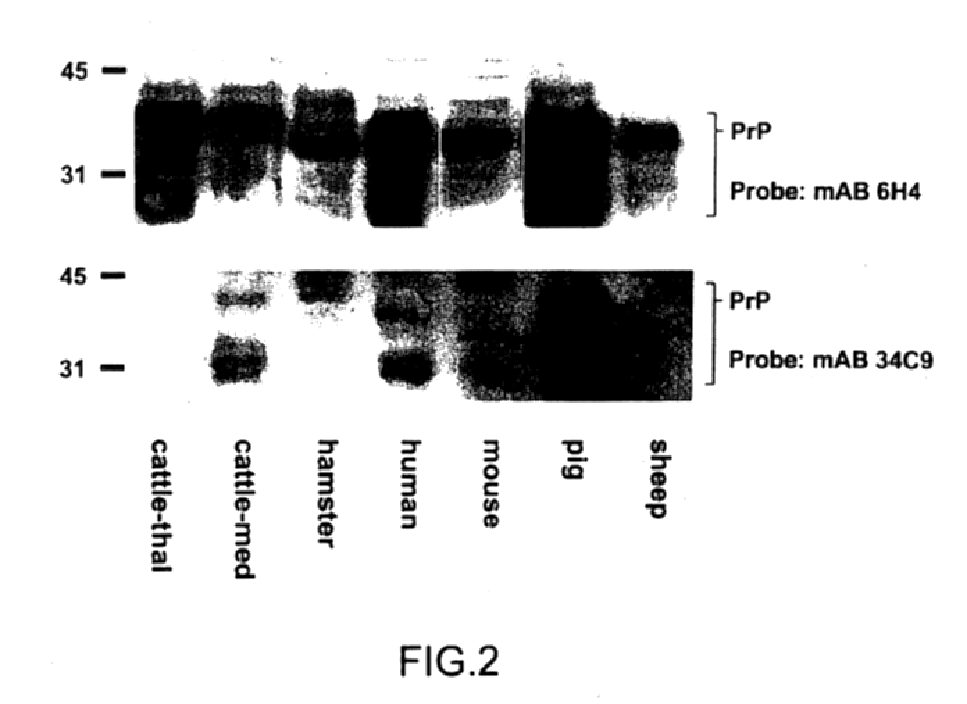
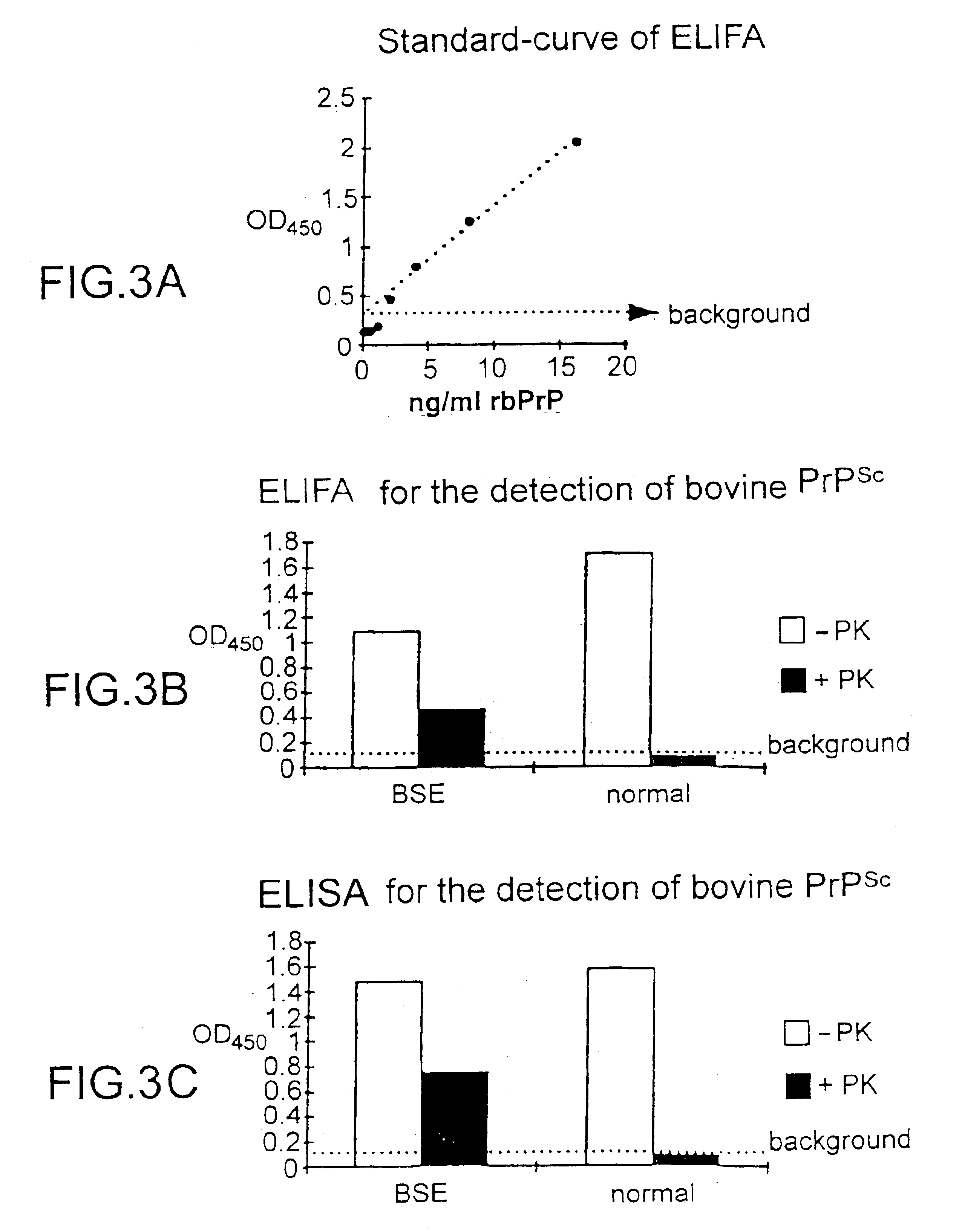
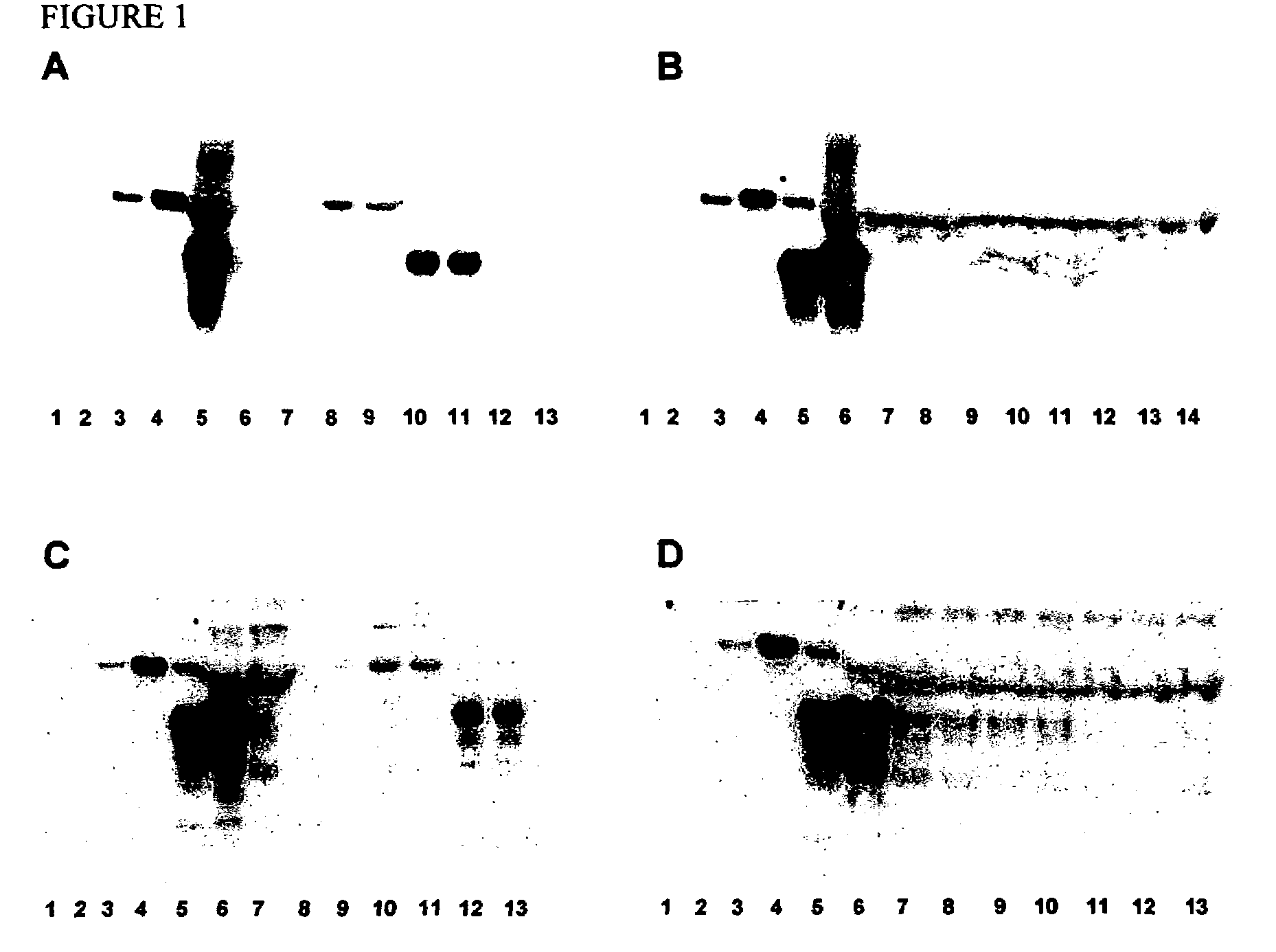
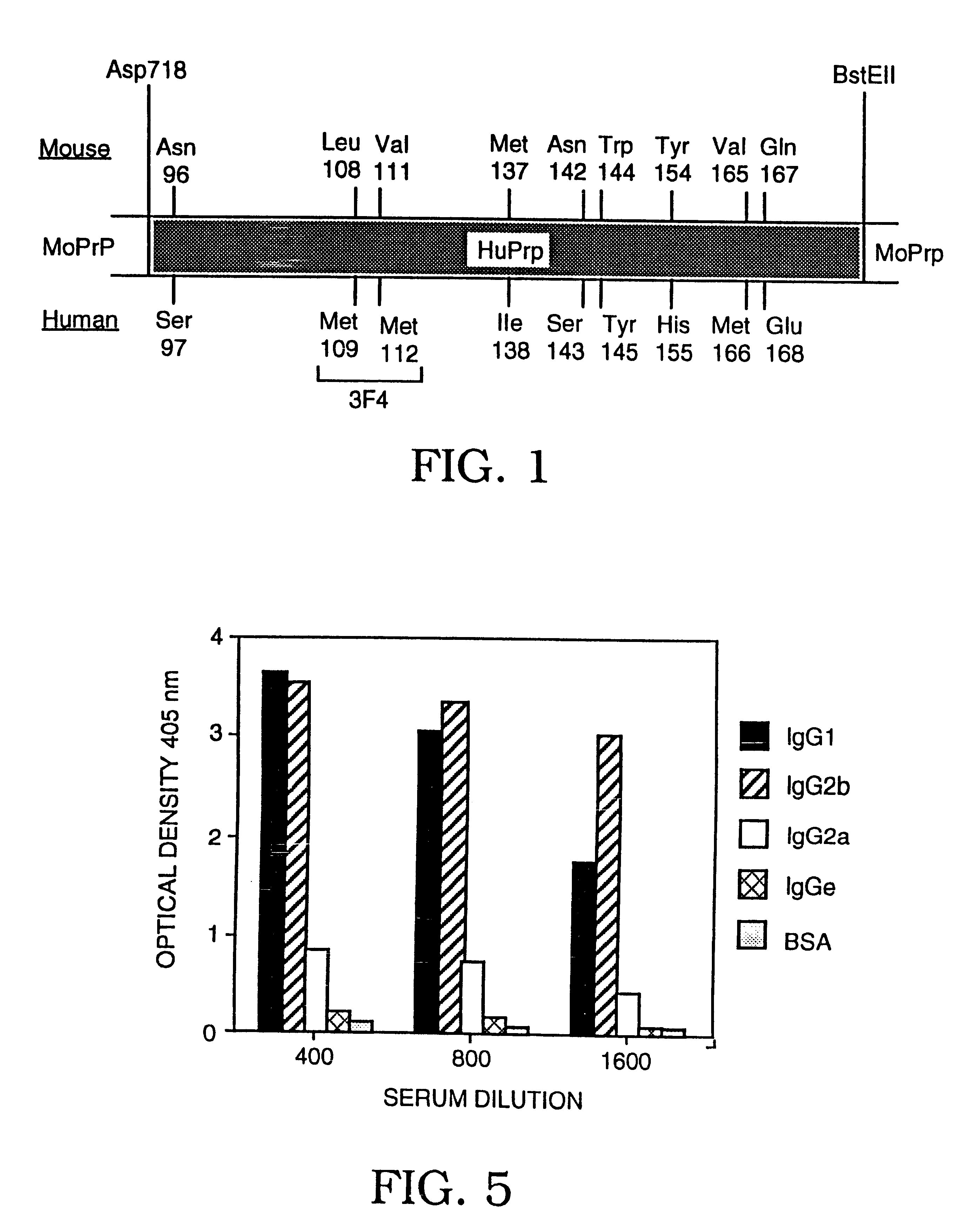
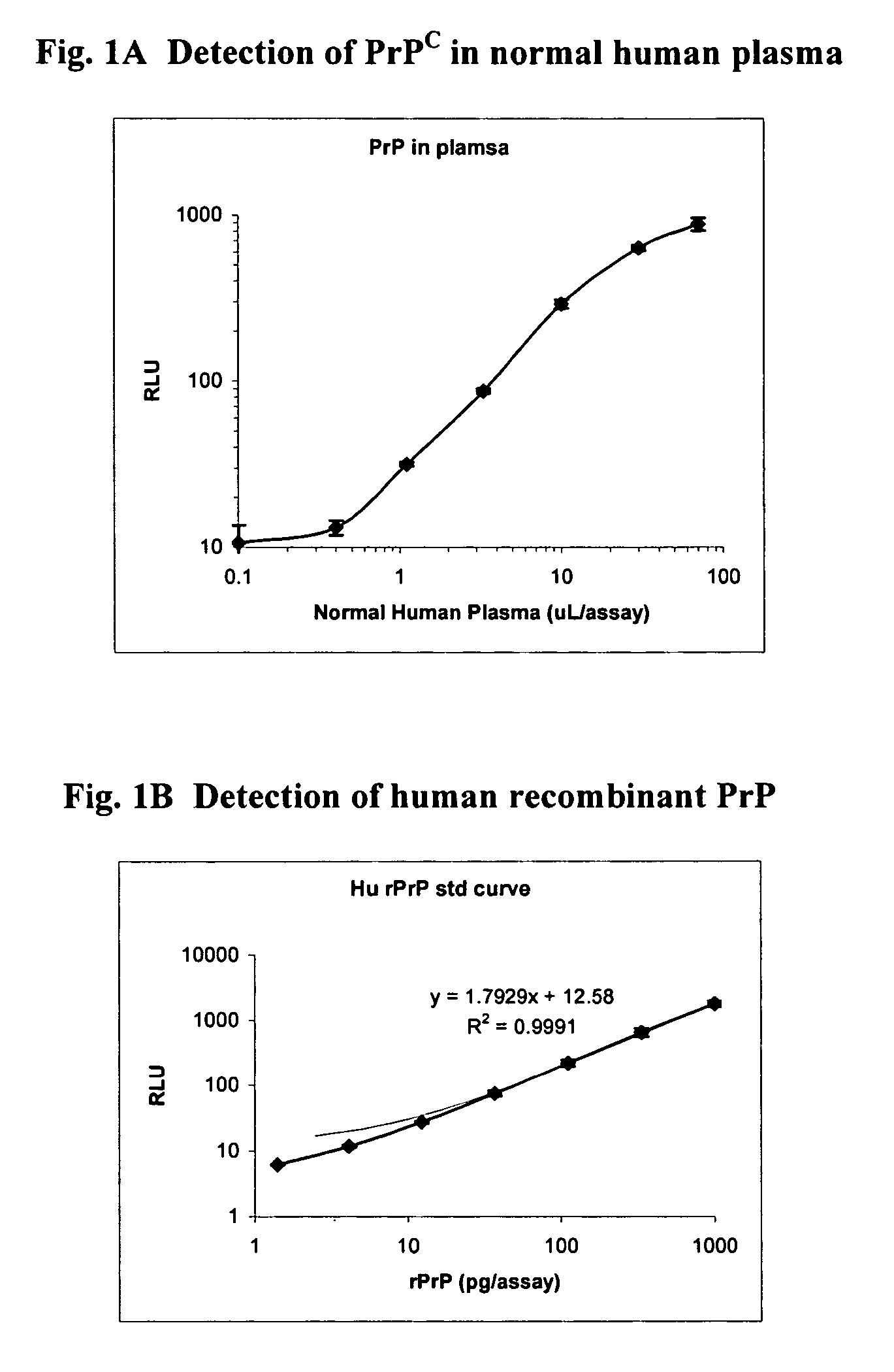
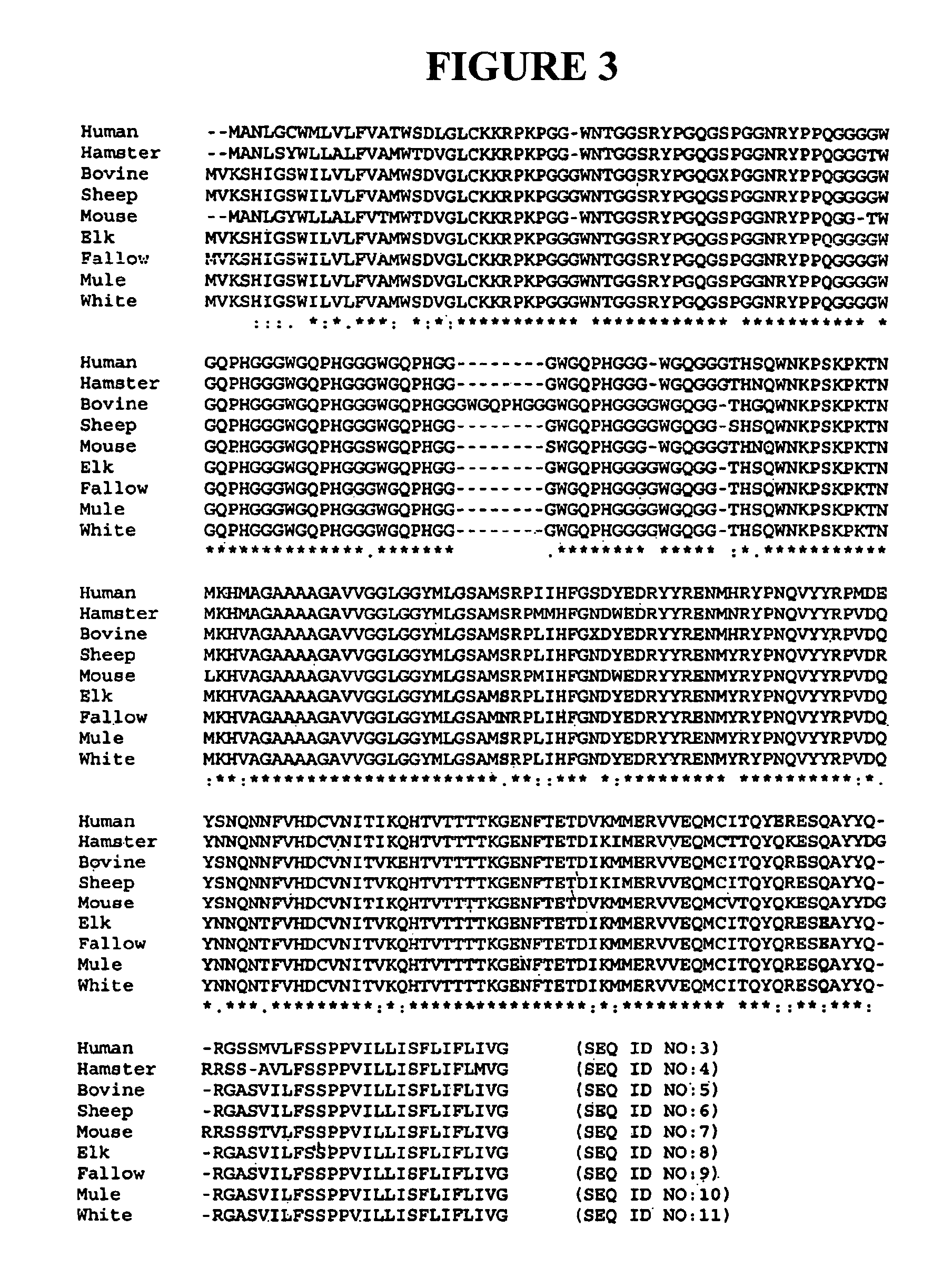
The presented invention relates to monoclonal antibodies useful in sensitive and specific immunological assays for the identification of prions in various tissues and body fluids, the production of such monoclonal antibodies by means of immunization of PrP<0 / 0 >mice by means of a new recombinant fragment of PrP and the use of the antibodies, e.g. for therapeutic and preventive treatments of humans and animals suffering from prion diseases.
Owner:UNIV ZURICH
Coatings
ActiveUS20060150862A1Group 4/14 element organic compoundsNon-macromolecular adhesive additivesCoated surfaceMedicine
This invention relates to coating a surface wherein the coated surface inhibits foulants such as cell and / or protein and / or prion adhesion or formation. In particular, the coated surface may be part of a medical device which inhibits bacterial adhesion and colonisation, thrombus formation and / or prion, blood protein and / or protein formation.
Owner:DUNDEE UNIV OF THE UNIV COURT OF THE
Catalytic monoclonal antibodies with protease activity for selective lysis of protein component of plaques aggregates pathological conditions
InactiveUS6387674B1Reduce usageUseful in treatmentNervous disorderPeptide/protein ingredientsAbzymeDisease
Catalytic monoclonal antibodies (abzymes) with selective protease activity in the pathologies characterized by the presence of plaques and fibrillar aggregates with protein component; methods for the preparation thereof and the use thereof as medicaments in the treatment of pathologies such as Alzheimer's disease, amyloidosis, atherosclerosis, prions diseases.
Owner:ABIOGEN PHARMA SPA
Cerebrospinal Fluid Purification System
The present invention provides methods and systems for conditioning cerebrospinal fluid (CSF). The methods provide for efficiently removing target compounds from CSF. The systems provide for a multilumen flow path and exchange of a majority volume portion of CSF in the CSF space. The removal and / or delivery of specific compounds can be tailored to the pathology of the specific disease. The removal is targeted and specific, for example, through the use of specific size-exclusion thresholds, antibodies against specific toxins, and other chromatographic techniques, as well as delivery and / or removal of targeted therapeutic agents. The invention finds use as a diagnostic, therapeutic and drug delivery platform for a variety of diseases affecting the CNS by accessing the CSF space. Exemplified disease conditions treatable by the present CSF processing systems and methods include, but are not limited to: Cerebral Vasospasm, Guillain Bane Syndrome, illustrating multi-lumen lumbar approach Alzheimer's, Parkinson's, Huntington's, Multiple Sclerosis, Amyotrophic Lateral Sclerosis, Spinal Cord Injury, Traumatic Brain Injury, Stroke, Cancer affecting the brain or spinal cord, Prion disease, Encephalitis from various causes, Meningitis from various causes, diseases secondary to enzymatic or metabolic imbalances, Biological Warfare, etc. For the first time, the present invention offers patients a disease-modifying, disruptive technology treatment platform that addresses the known disease pathogenesis of a number of neurologic conditions to which there are presently limited and ineffective treatment options.
Owner:NEUROFLUIDICS
Inhibitors and disassemblers of fibrillogenesis
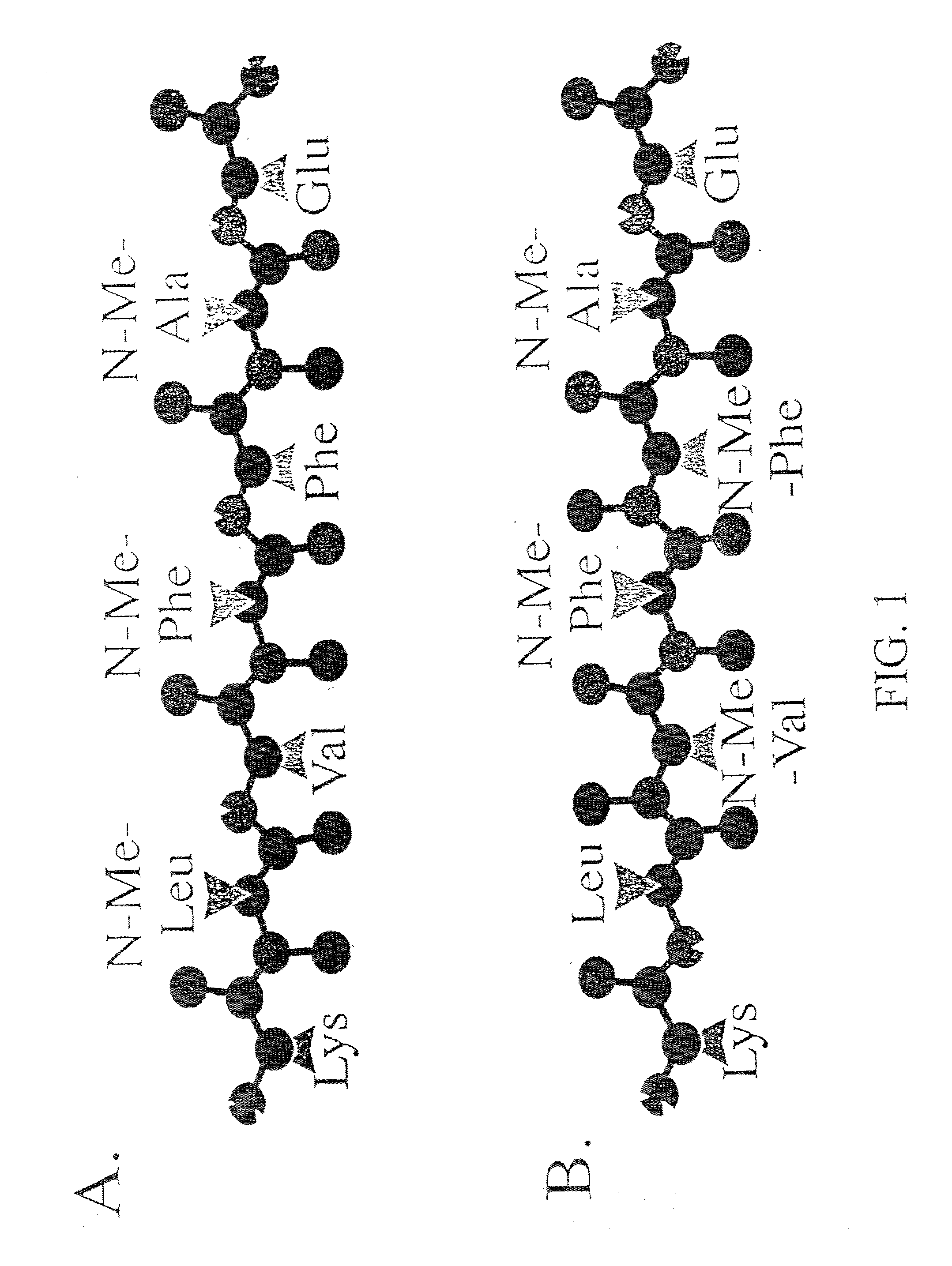
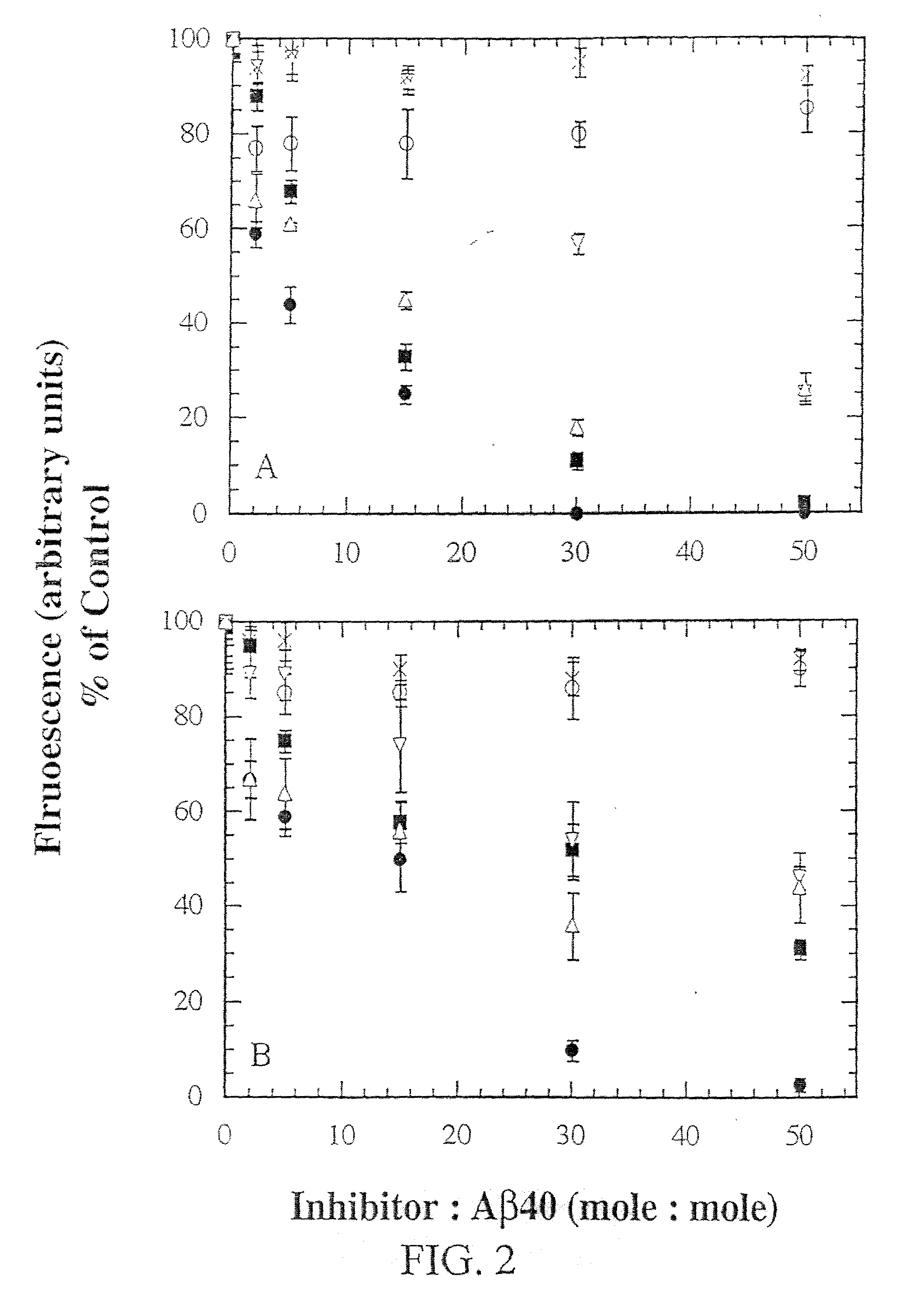
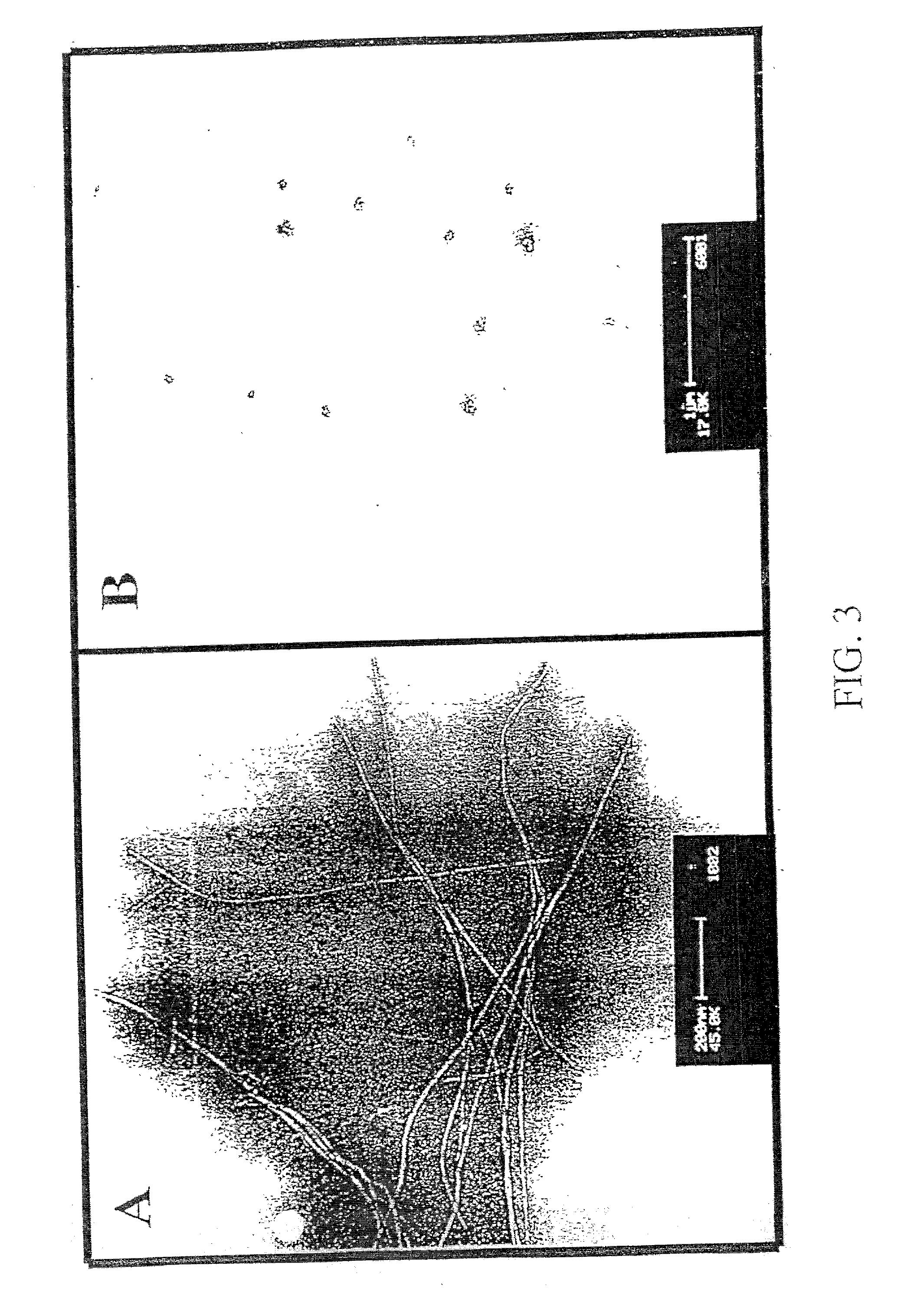
InactiveUS20030130484A1Highly effective at disassembling pre-formed fibrilsInhibition formationPeptide/protein ingredientsPeptide preparation methodsFiberAmino acid
Methods and compositions are presented that inhibit fibril formation and / or bring about disassembly of pre-formed fibrils. Compositions include peptides with short beta-strands with two faces: one that can bind to beta-amyloids through hydrogen bonds, and one which blocks propagation of hydrogen bonding needed to form fibrils. Thus, short congeners of the fibril protein containing N-methyl amino acids or esters are provided for the inhibition of fibril formation and for the disassembly of pre-existing or pre-formed fibrils. Specific aspects address beta-amyloid fibrils; prion mediated fibrils; Huntington protein fibrils. Methods for screening for potential fibril inhibitors and disassemblers, diagnostic analysis and treatments are provided.
Owner:UNIVERSITY OF CHICAGO
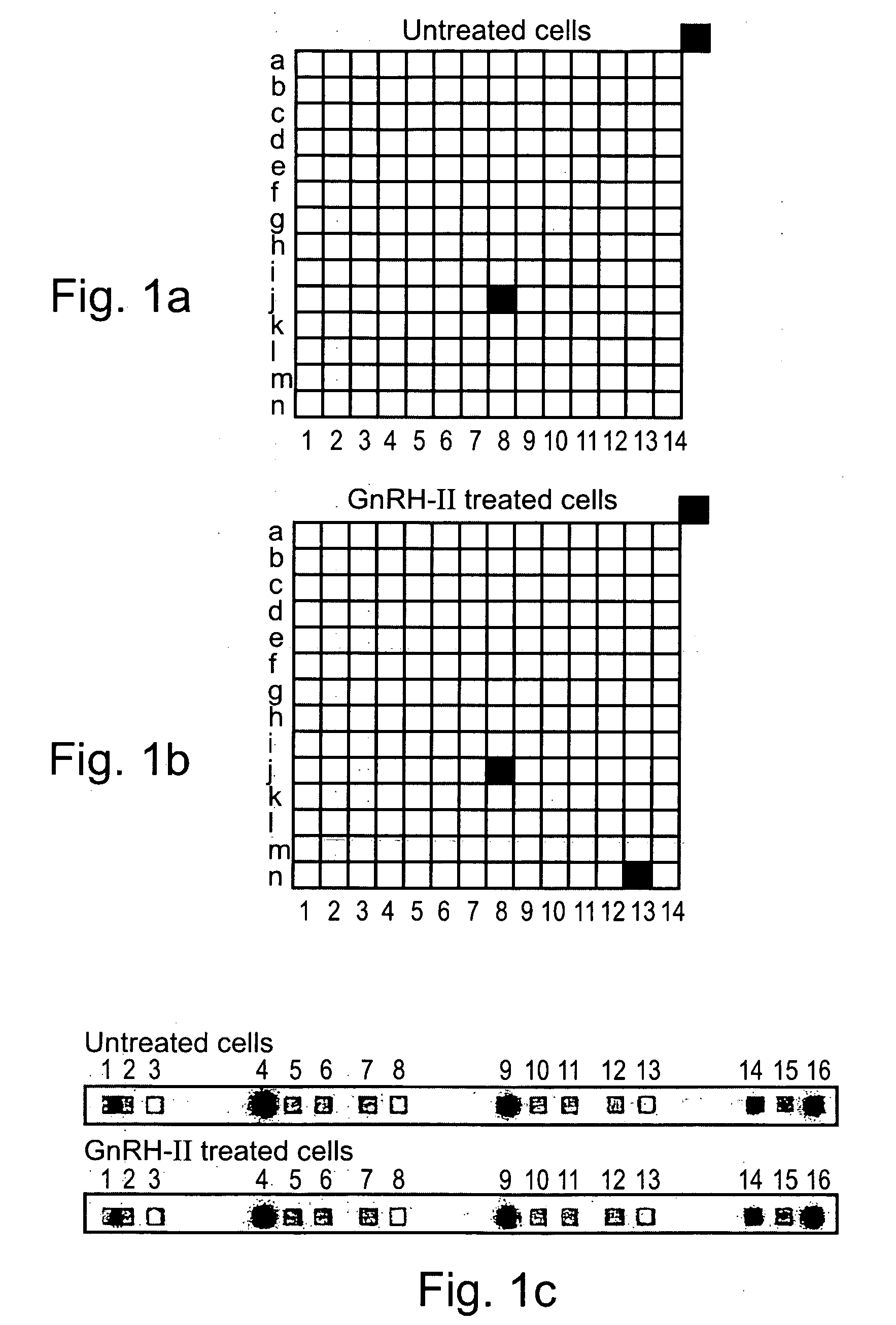
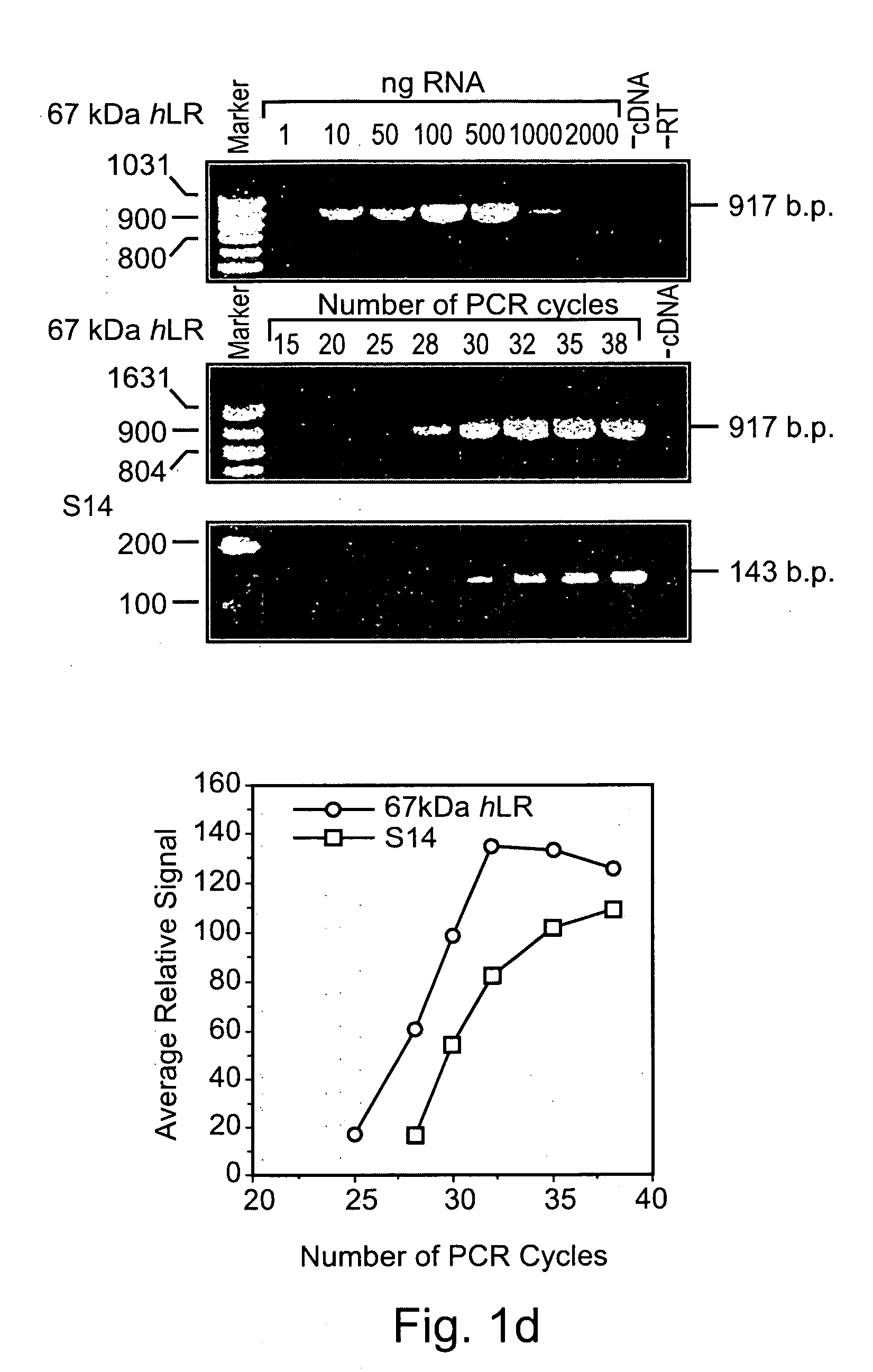
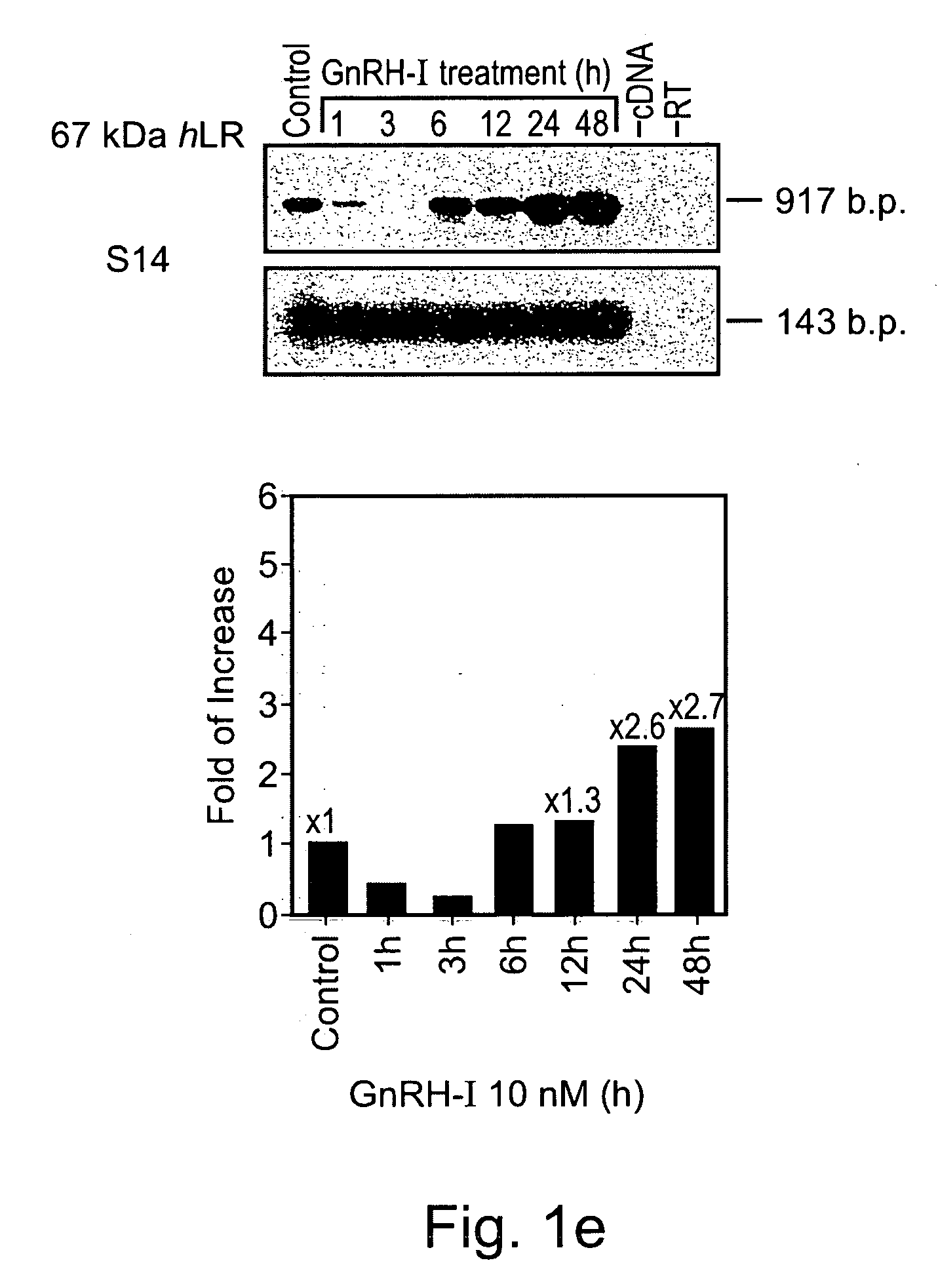
Methods and pharmaceutical compositions for gnrh-II and gnrh-II modulation of t-cell activity, adhesion, migration and extravasation
Methods and compositions comprising GnRH-I and GnRH-II, GnRH-I and GnRH-II antibodies, anti-receptor antibodies, polynucleotide constructs and GnRH-I and GnRH-II analogs for immune enhancement and suppression, prevention and treatment of diseases and conditions characterized by abnormal T-cell activity, treatment of viral and prion-related diseases, and treatment of T-cell related neoplastic diseases are disclosed.
Owner:KOCH YITZHAK PROF +1
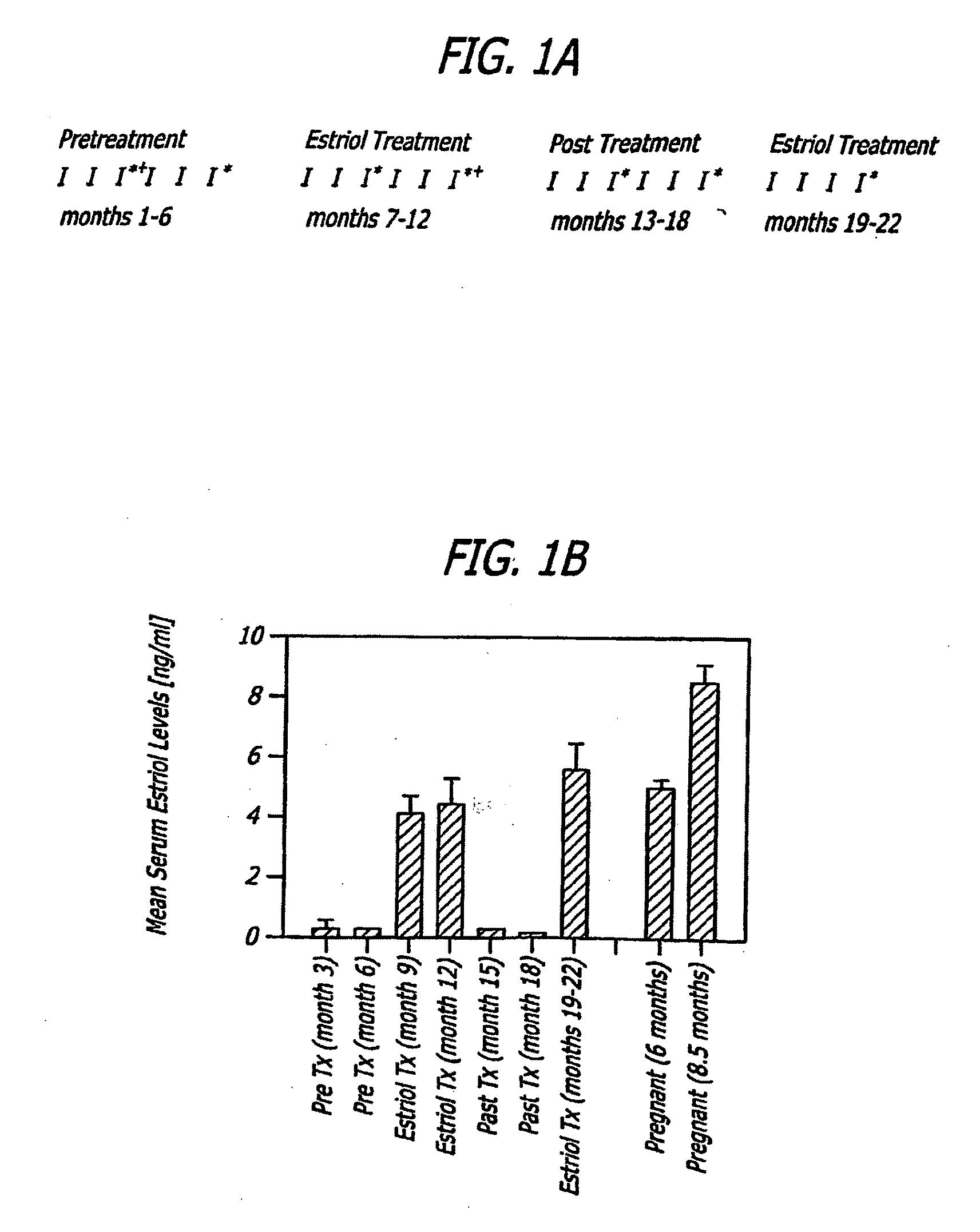
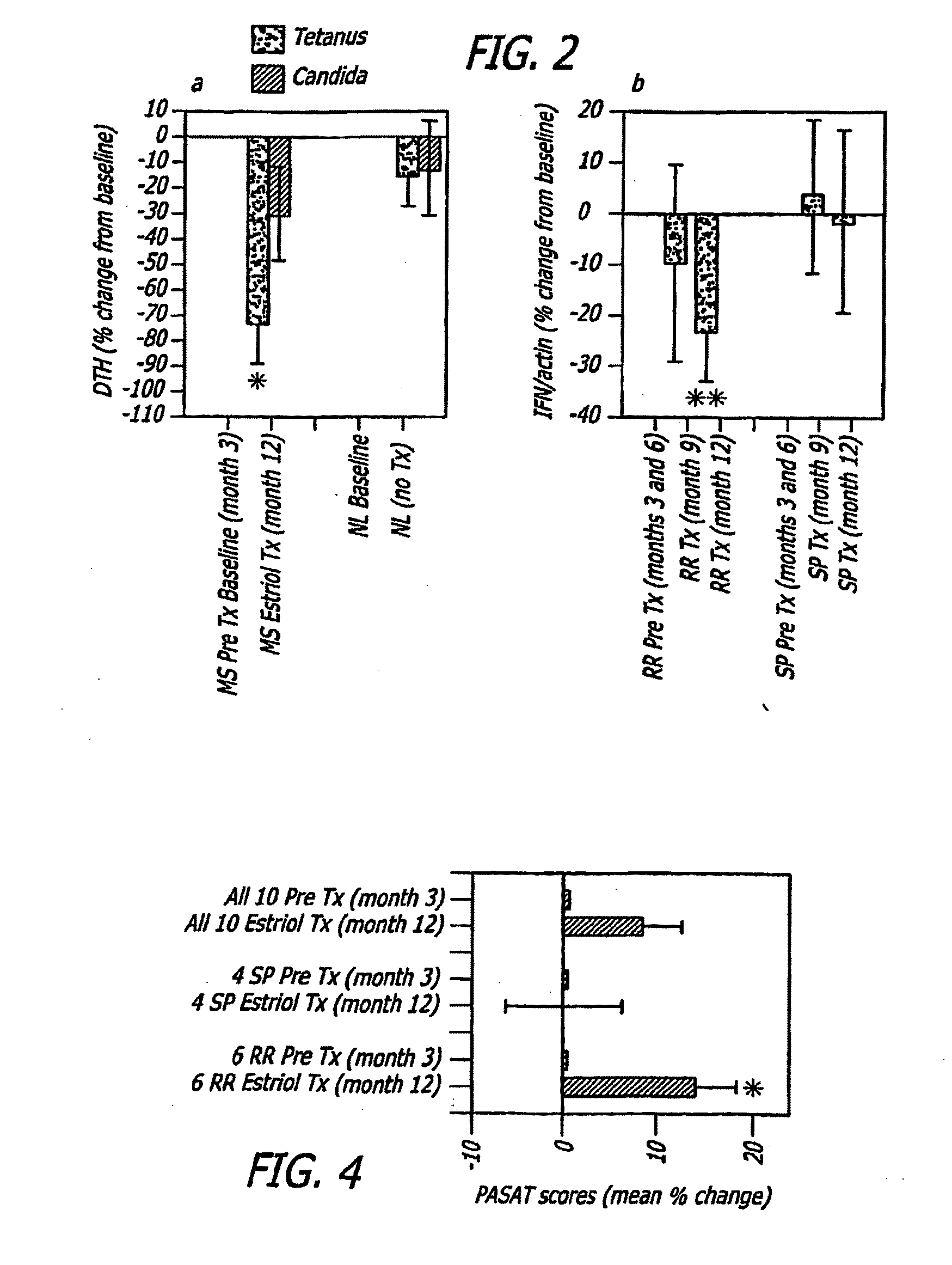
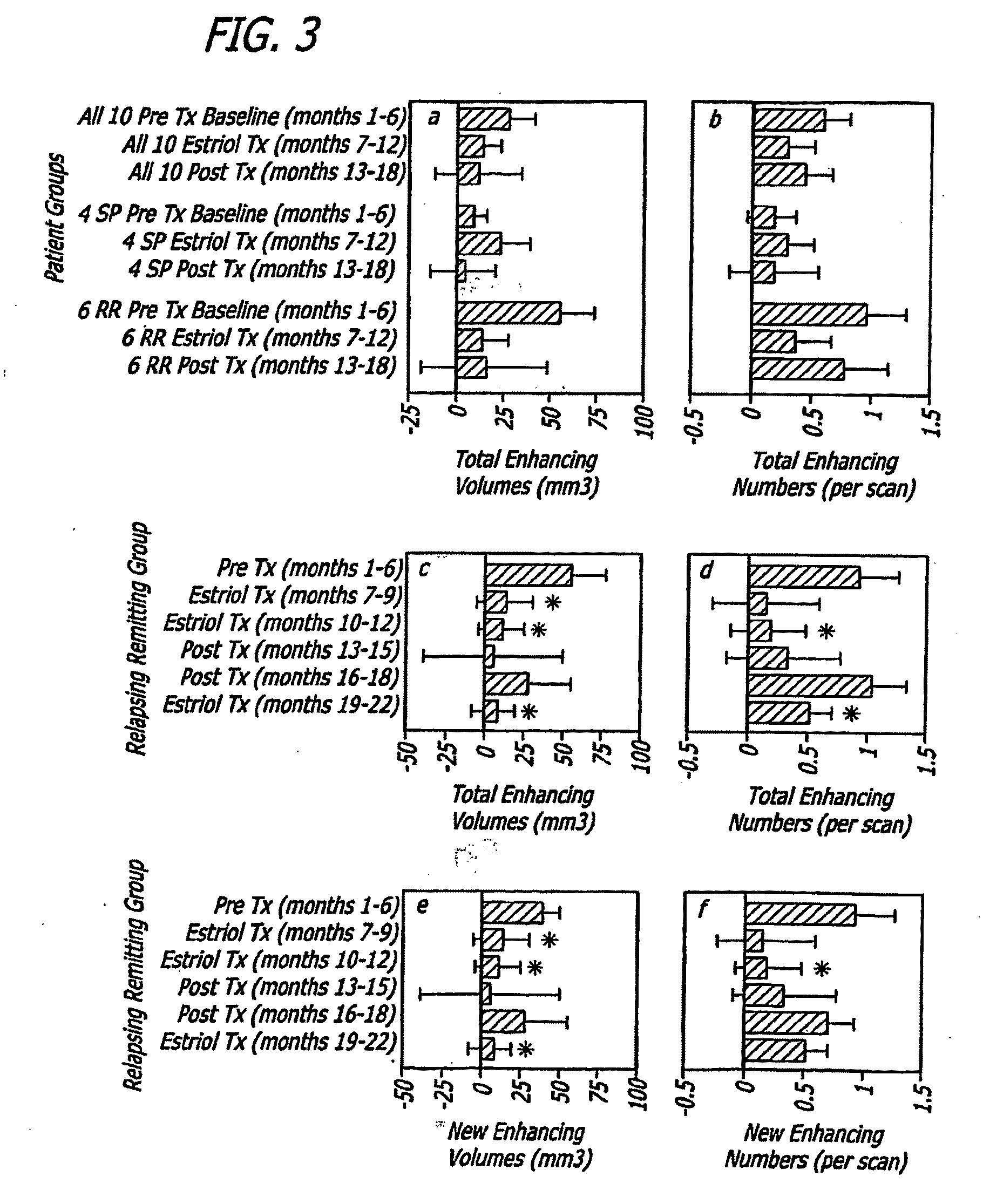
Estriol Therapy for Autoimmune and Neurodegenerative Diseases and Disorders
The present invention discloses administering steroid hormones to mammals to treat autoimmune related diseases, neurodegenerative diseases or disorders, such as Alzheimer's disease, Parkinson's Disease, multiple sclerosis, stroke, ALS Pick's disease, prion disease and Huntington's disease. Most preferably the invention uses estrogens, estranges, estriol or estrogen receptor active agents to prevent or ameliorate clinical symptoms of the diseases and disorders.
Owner:RGT UNIV OF CALIFORNIA
EB Matrix Production from Fetal Tissues and its Use for Tissue Repair
InactiveUS20020146393A1Reducing biological propertyPreserve strengthBiocideHepatocytesCross-linkTissue repair
<heading lvl="0">Abstract of Disclosure< / heading> A method of forming and preserving a bioremodelable, biopolymer scaffold material by subjecting animal tissue, particularly fetal or neo-natal tissue, to chemical and mechanical processing. The process includes, but is not limited t, harvesting the tissue, optionally extracting growth and differentiation factors from the tissue, inactivating infective agents of the tissue, mechanically expressig undesirable components frm the tissue, delipidizing the tissue, washing the tissue, optionally drying the tissue, and optionally cross-linking the tissue not necessarily in the order described. The resulting product, EBM, is characterized by its microbial, fungal, viral and prion inactivated state. EBM is storng, bioremodelable, drapable and does not undergo calicification. EBM supplants previous inventions because of its unique method of preparation and broad applicability in tissue reengineering
Owner:TEI BIOSCI
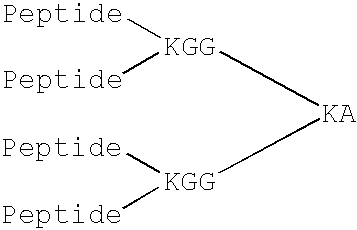
Prion protein binding materials and methods of use
ActiveUS7393658B2Viral antigen ingredientsBiological testingHamsterProteinaceous infectious particle
Prion protein binding materials and methods for using the binding materials to detect or remove a prion protein from a sample, such as a biological fluid or an environmental sample. The binding materials are capable of binding to one or more forms of prion protein including cellular prion protein (PrPc), infectious prion protein (PrPsc), recombinant prion protein (PrPr), and proteinase resistant prion protein (PrPres). Prions from various species, including humans and hamsters, are bound by the binding materials.
Owner:PATHOGEN REMOVAL & DIAGNOSTIC TECH +1
Synthetic immunogenic but non-deposit-forming polypeptides and peptides homologous to amyloid beta, prion protein, amylin, alpha-synuclein, or polyglutamine repeats for induction of an immune response thereto
InactiveUS7479482B2Reduce formationAvoid formingHormone peptidesNervous disorderPassive ImmunizationsAmyloid beta
The present invention relates to immunogenic but non-depositing-forming polypeptides or peptides homologous to amyloid β, prion, amylin or α-synuclein which can be used alone or conjugated to an immunostimulatory molecule in an immunizing composition for inducing an immune response to amyloid β peptides and amyloid deposits, to prion protein and prion deposits, to amylin and amylin deposits, to α-synuclein and deposits containing α-synuclein, or to polyglutamine repeats and deposits of proteins containing polyglutamine repeats. Described are also antibodies directed against such peptides, their generation, and their use in methods of passive immunization to such peptides and deposits.
Owner:NEW YORK UNIV
Methods and compositions for the treatment of symptoms of prion diseases
InactiveUS9061033B2Peptide/protein ingredientsMicrobiological testing/measurementDiseaseFaecal chymotrypsin
A therapeutic composition for the treatment of the symptoms of prion diseases and the method for preparing the therapeutic agents is disclosed. The therapeutic composition is a stable pharmaceutical composition comprising one or more digestive and / or pancreatic enzymes. The therapeutic composition may be manufactured by a variety of encapsulation technologies. Delivery of the therapeutic composition may be made orally, through injection, by adherence of a medicated patch or other method. Further, a method of using fecal chymotrypsin level as a biomarker for the presence of a prion disease, or the likelihood of an individual to develop a prion disease is disclosed.
Owner:CUREMARK
Detection of conformationally altered proteins and prions
InactiveUS20080171341A1Reliable and and safe methodRapid diagnosisPeptide/protein ingredientsDepsipeptidesProteinaceous infectious particleDisease cause
Owner:ADLYFE INC
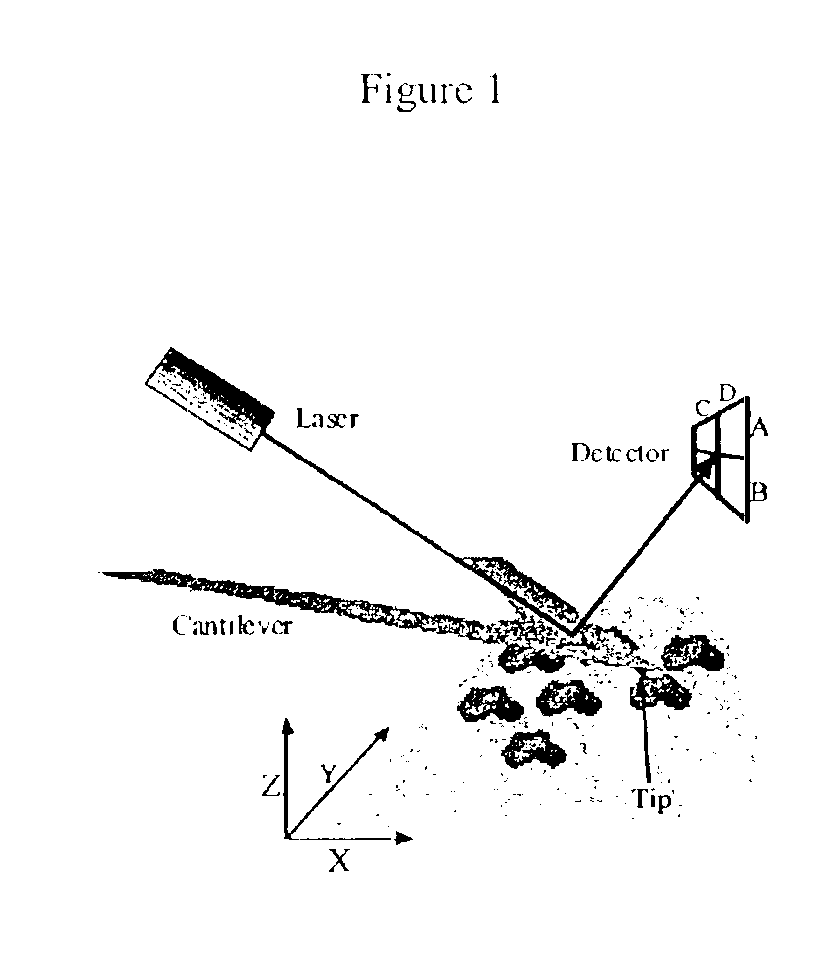
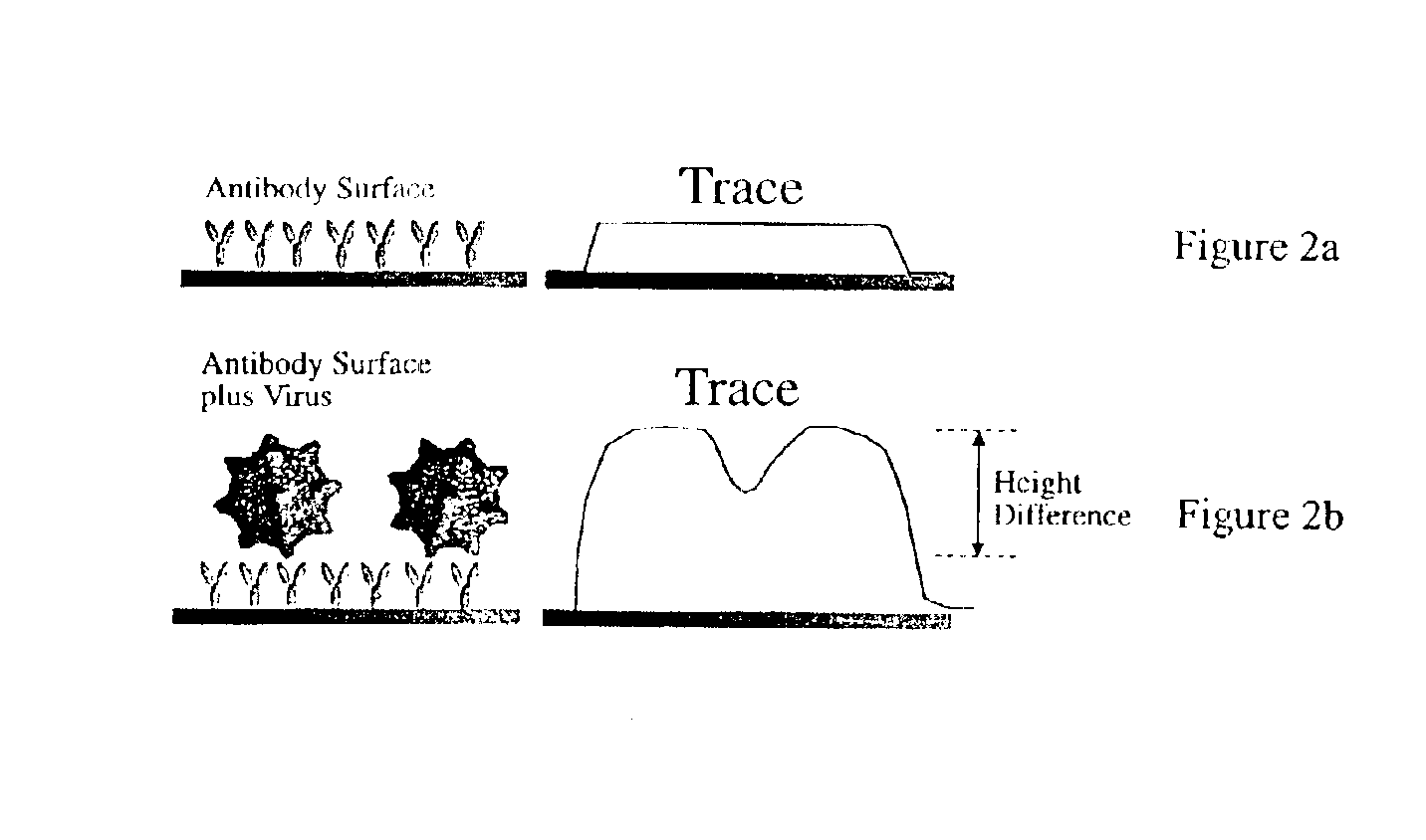
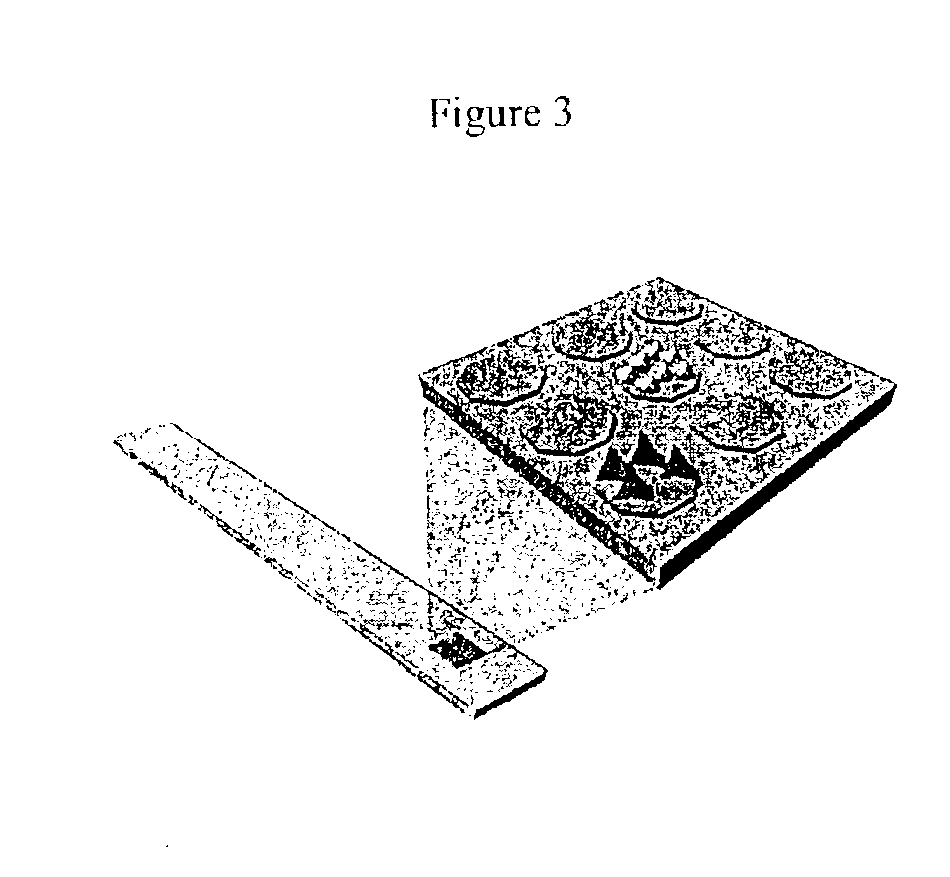
Device and method of use for detection and characterization of pathogens and biological materials
The present invention includes a method and apparatus for the detection of a target material. The method and apparatus includes providing a substrate with a surface and forming a domains of deposited materials thereon. The deposited material can be placed on the surface and bound directly and non-specifically to the surface, or it may be specifically or non-specifically bound to the surface. The deposited material has an affinity for a specific target material. The domains thus created are termed affinity domains or deposition domains. Multiple affinity domains of deposited materials can be deposited on a single surface, creating a plurality of specific binding affinity domains for a plurality of target materials. Target materials may include, for example, pathogens or pathogenic markers such as viruses, bacteria, bacterial spores, parasites, prions, fungi, mold or pollen spores. The device thus created is incubated with a test solution, gas or other supporting environment suspected of containing one or more of the target materials. Specific binding interactions between the target materials and a particular affinity domain occurs and is detected by various methods.
Owner:BIOFORCE NANOSCI
Prion protein binding materials and methods of use
ActiveUS7510848B2Bacterial antigen ingredientsViral antigen ingredientsHamsterProteinaceous infectious particle
Prion protein binding materials and methods for using the binding materials to detect or remove a prion protein from a sample, such as a biological fluid or an environmental sample. The binding materials are capable of binding to one or more forms of prion protein including cellular prion protein (PrPc), infectious prion protein (PrPsc), recombinant prion protein (PrPr), and proteinase resistant prion protein (PrPres). Prions from various species, including humans and hamsters, are bound by the binding materials.
Owner:PATHOGEN REMOVAL & DIAGNOSTIC TECH +1
Antibodies specific for native PrPSc
InactiveUS6372214B1Fast and efficient cost-effective assayFast and efficientImmunoglobulins against animals/humansHybrid cell preparationDiseaseMammal
Owner:THE SCRIPPS RES INST
Prion-specific peptoid reagents
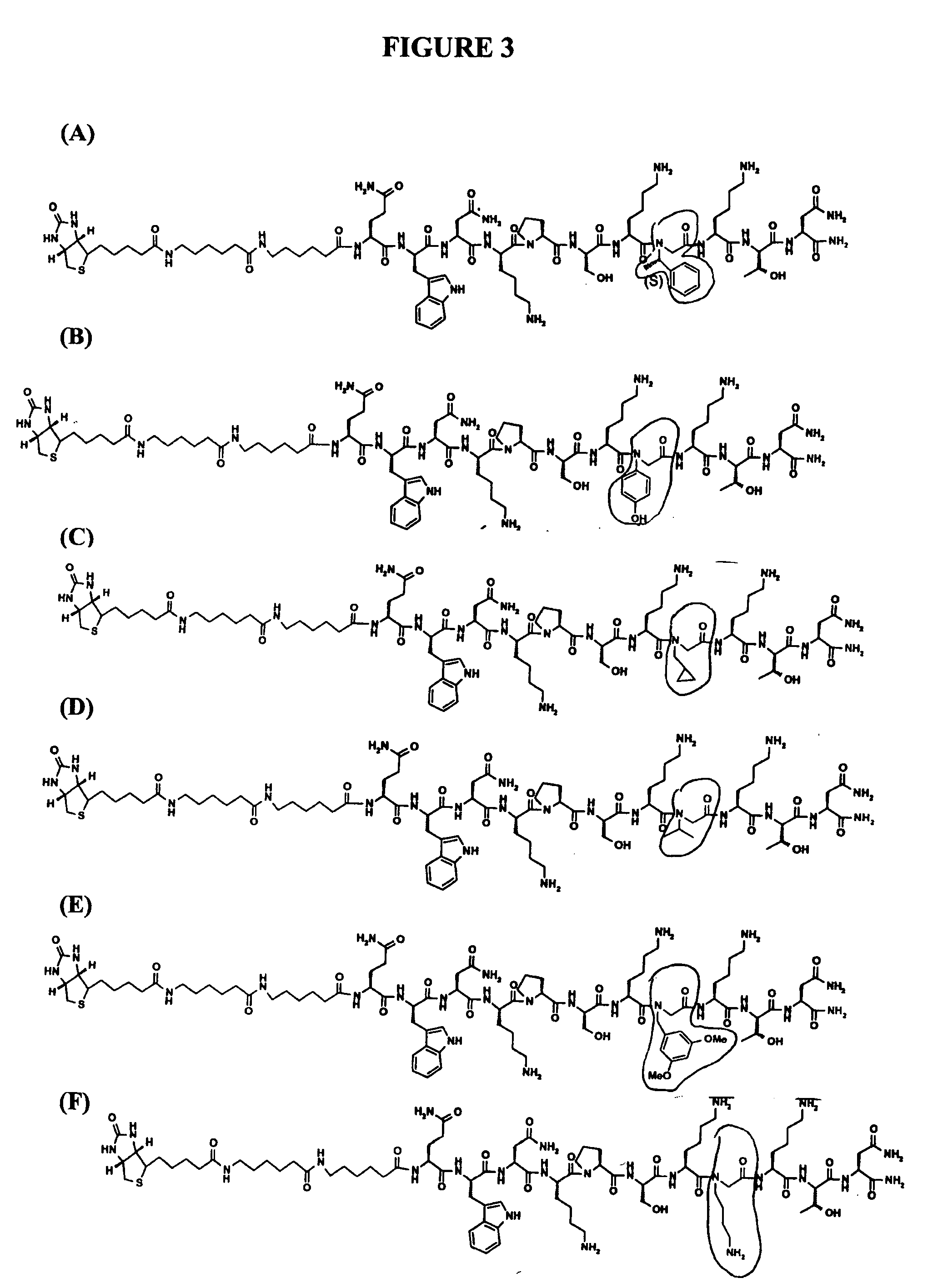
The invention relates to peptoid reagents that interact preferentially with a pathogenic form of a conformational disease protein as compared to a nonpathogenic form of the conformational disease protein where the peptoid reagent comprises an amino-terminal region, a carboxy-terminal region, and at least one peptoid region between the amino-terminal region and the carboxy-terminal region where the peptoid region comprises 3 to about 30 N-substituted glycines, and optionally one or more amino acids. The invention also relates to methods of using the peptoids in detecting and isolating prions, and in the treatment and prevention of prion-related diseases.
Owner:NOVARTIS AG
Prion-specific peptide reagents
InactiveUS20050118645A1Nervous disorderPeptide/protein ingredientsProteinaceous infectious particleAntibody
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
Methods of sterilizing biological mixtures using stabilizer mixtures
InactiveUS20050106728A1Reducing residual solvent contentEffective protectionBiocideDead animal preservationFungal microorganismsBiological materials
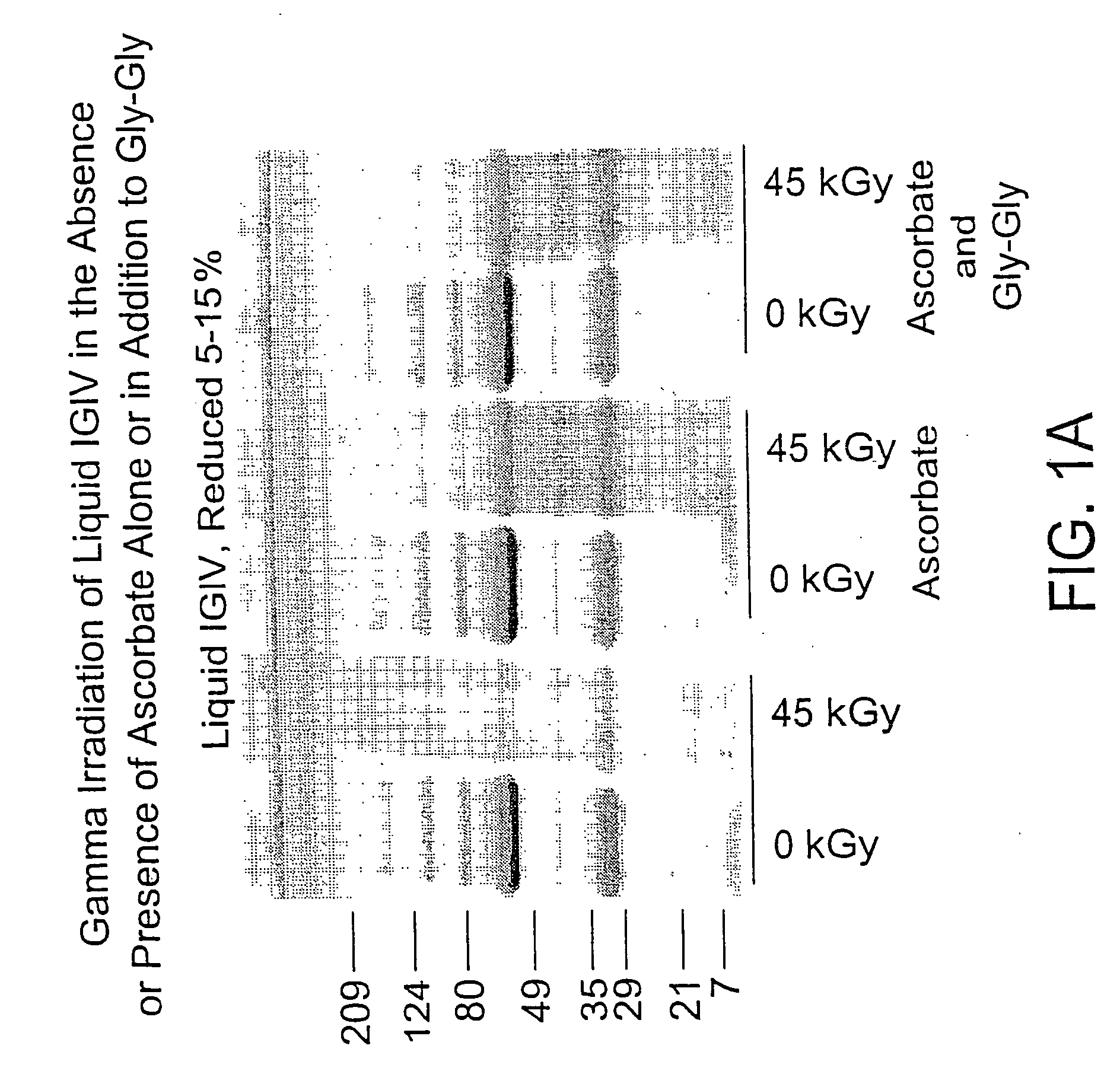
Methods are disclosed for sterilizing biological materials to reduce the level of one or more biological contaminants or pathogens therein, such as viruses, bacteria (including inter- and intracellular bacteria, such as mycoplasmas, ureaplasmas, nanobacteria, chlamydia, rickettsias), yeasts, molds, fungi, single or multicellular parasites, and / or prions or similar agents responsible, alone or in combination, for TSEs. These methods involve the use of stabilizer mixtures in methods of sterilizing biological materials with irradiation.
Owner:CLEARANT
Nanoparticles for providing immune responses against infectious agents
ActiveUS20090297614A1Strong immune responseConvenience needsPowder deliveryHeavy metal active ingredientsNanoparticleT cell
Nanoparticles for providing immune responses for the treatment or prophylaxis of infection by infectious agents such as viruses, parasites, bacteria, prions and fungi are described which comprises a core including metal and / or semiconductor atoms, wherein the core is covalently linked to a plurality of ligands, the ligands including a carbohydrate residue capable of stimulating an innate immune response, a T cell helper peptide and a danger signal. This platform may then be adapted by including one or more further ligands capable of producing a specific response to a target infectious agent.
Owner:MIDATECH LTD
Compositions for treating amyloid associated diseases
InactiveUS7732479B2Easy to useInhibition formationBiocideNervous disorderWhole bodyProteinaceous infectious particle
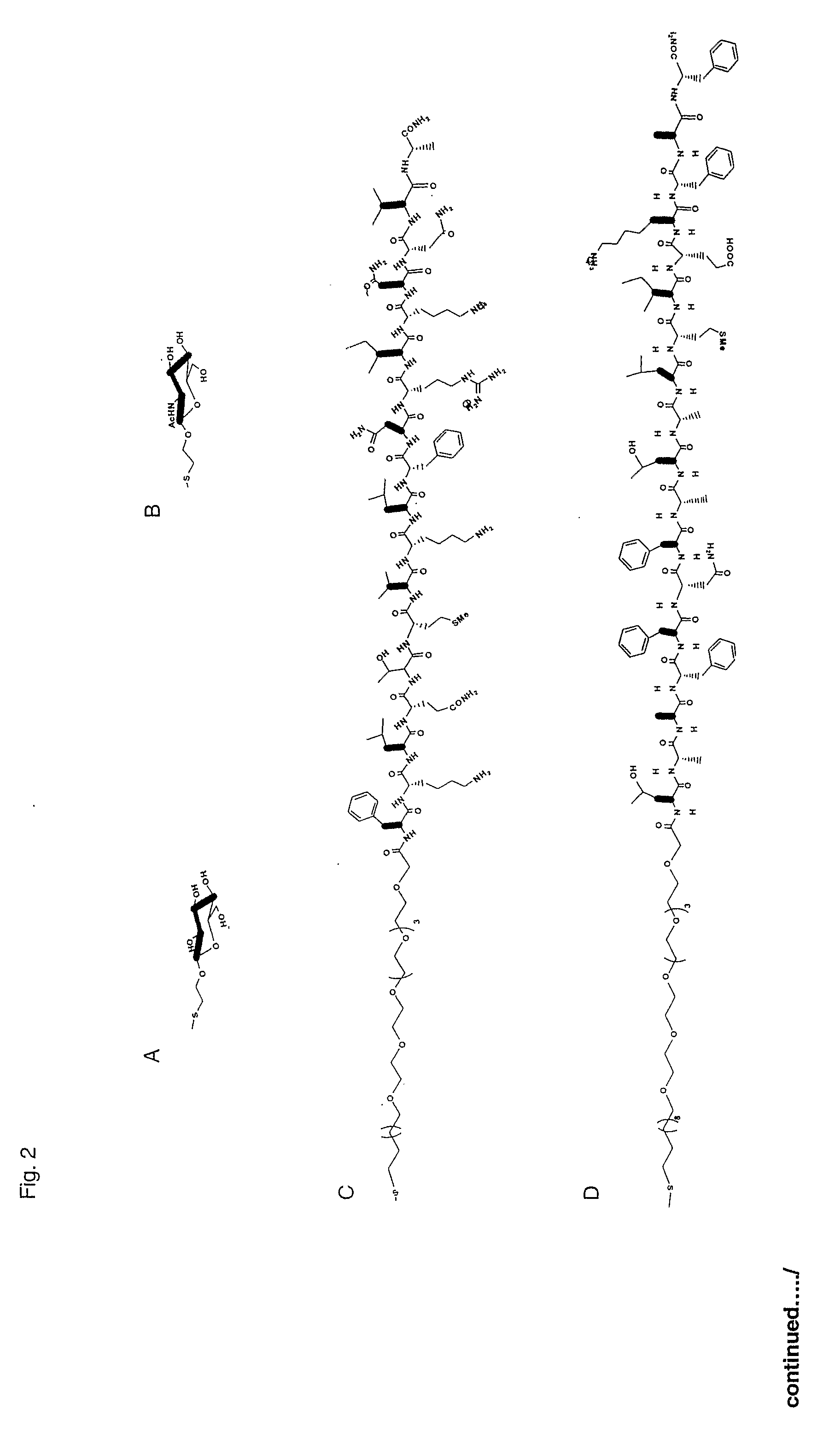
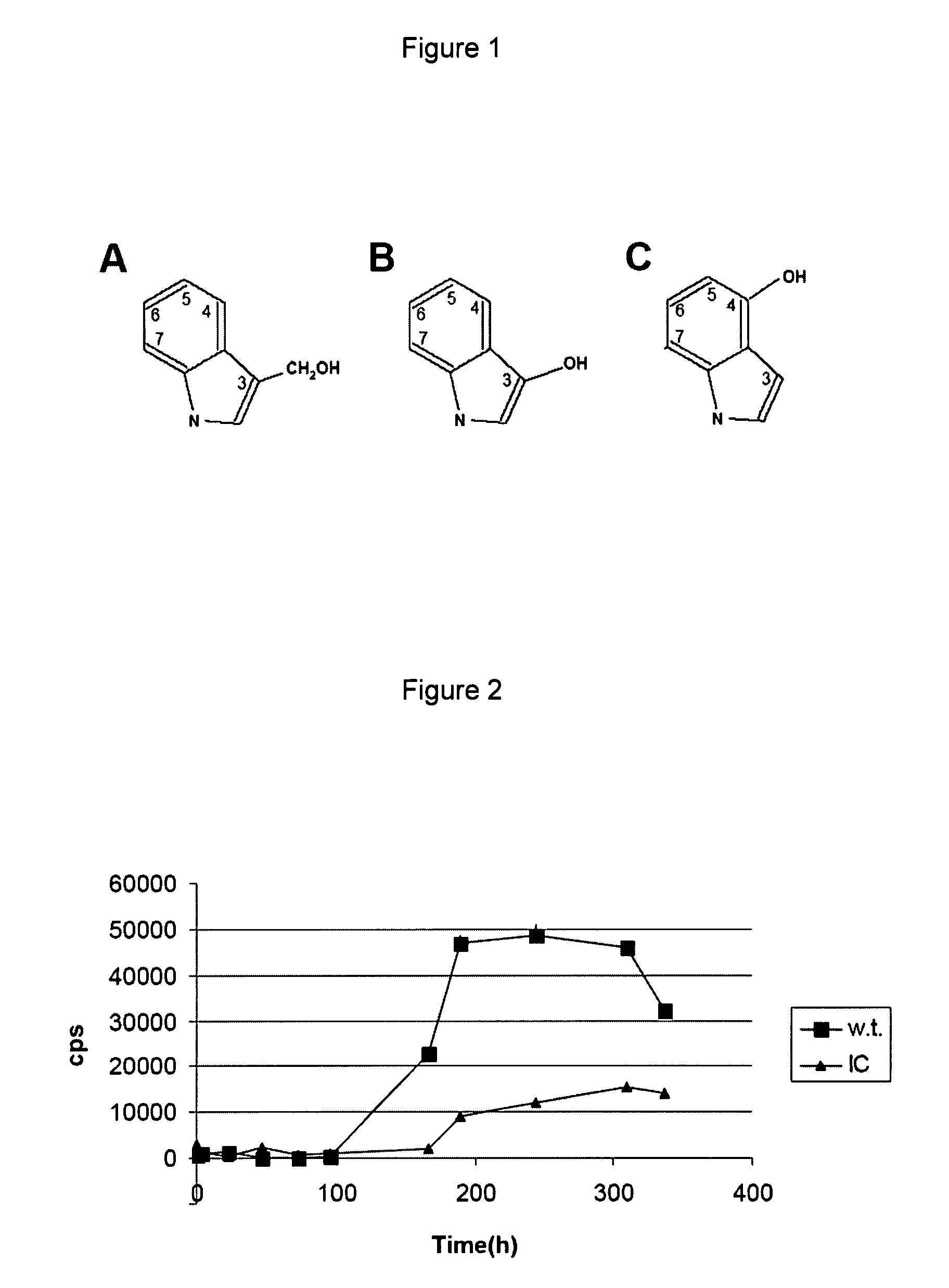
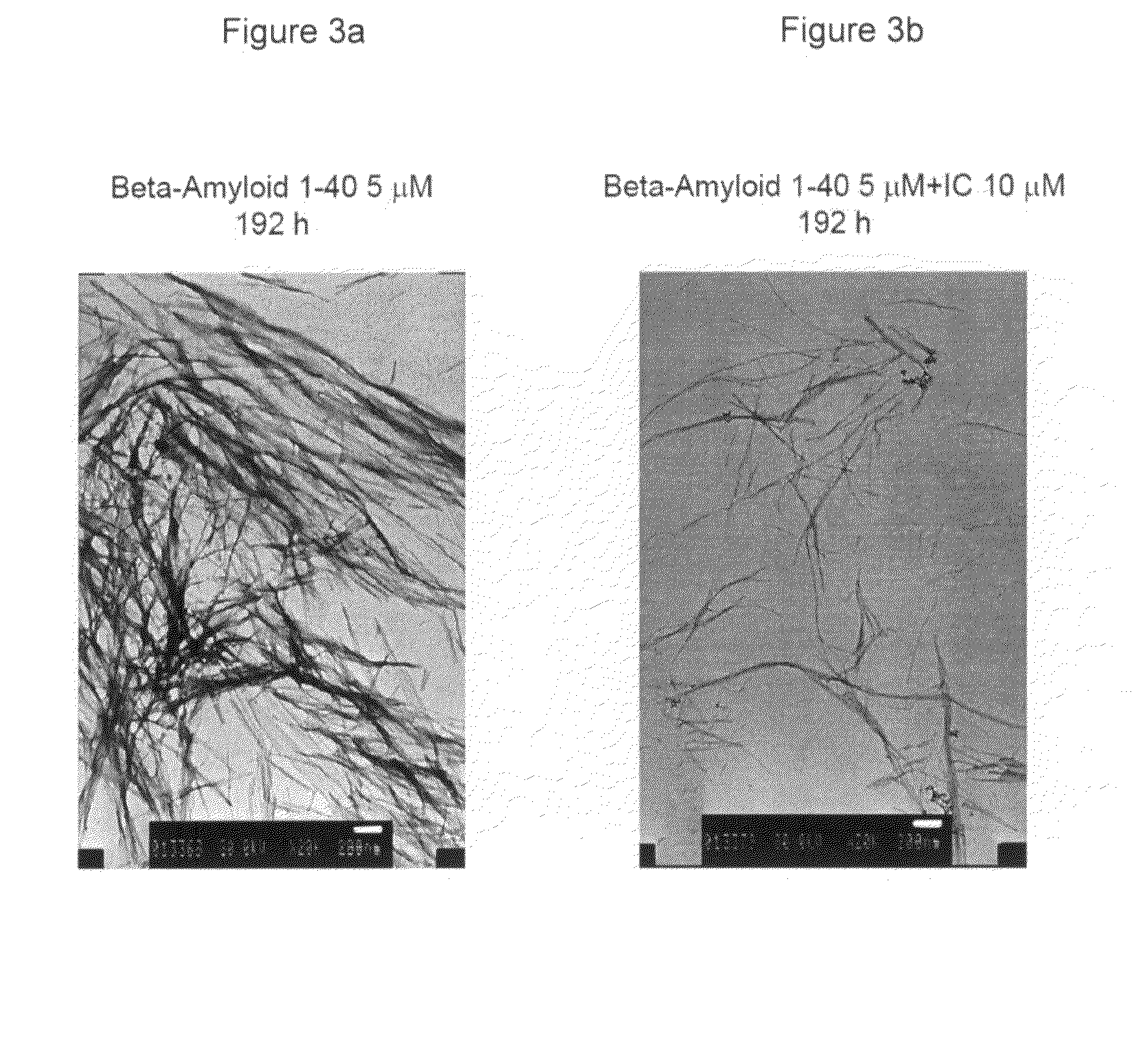
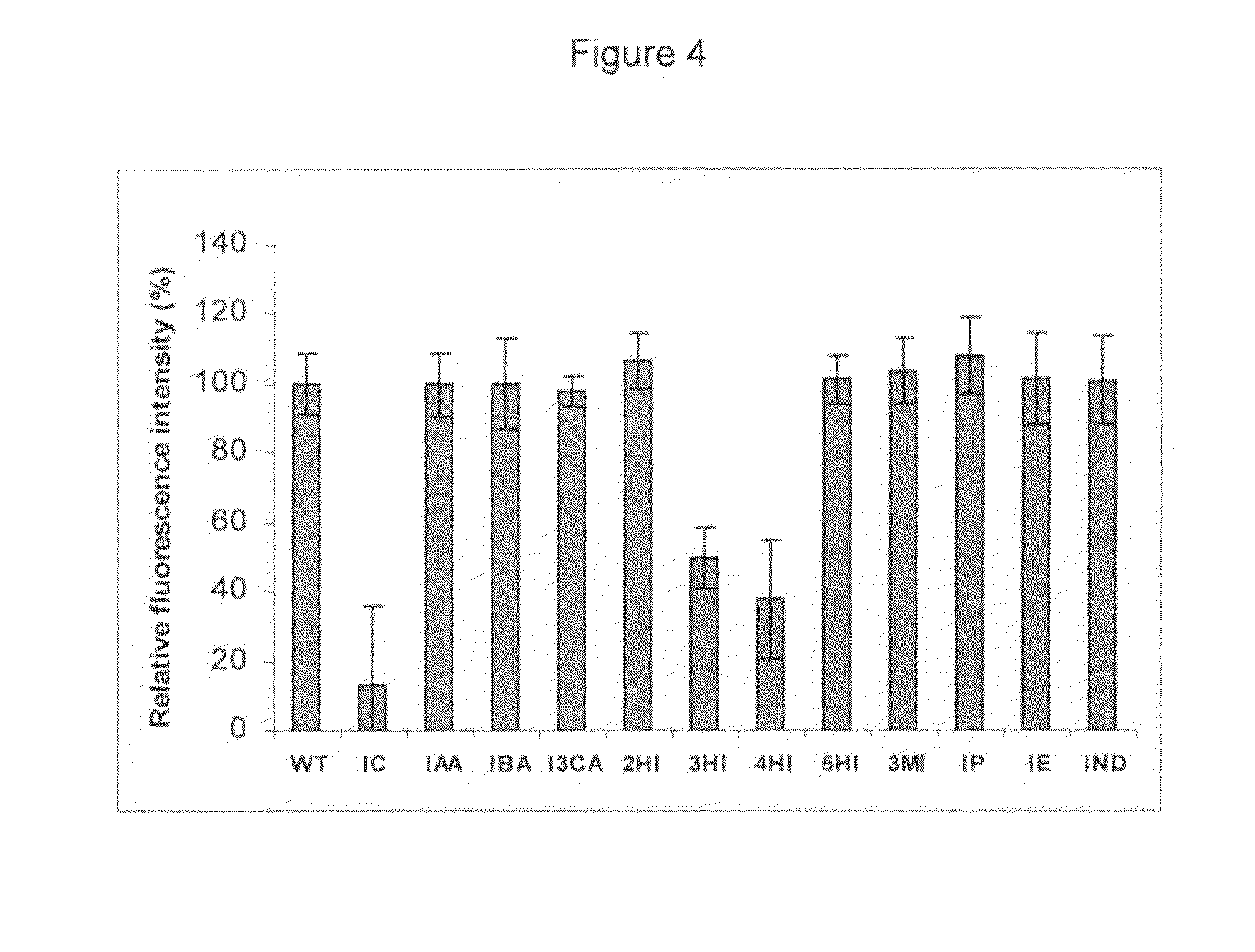
Indole derivatives, compositions including same, and methods utilizing same for the treatment of amyloid associated diseases, such as type II diabetes mellitus, Alzheimer's dementia or diseases, systemic and localized amyloidosis, and prion-related encephalopathies are provided.
Owner:TEL AVIV UNIV FUTURE TECH DEVMENT
Methods and compositions for the treatment of symptoms of prion diseases
InactiveUS20130195833A1Peptide/protein ingredientsMicrobiological testing/measurementDiseaseFaecal chymotrypsin
A therapeutic composition for the treatment of the symptoms of prion diseases and the method for preparing the therapeutic agents is disclosed. The therapeutic composition is a stable pharmaceutical composition comprising one or more digestive and / or pancreatic enzymes. The therapeutic composition may be manufactured by a variety of encapsulation technologies. Delivery of the therapeutic composition may be made orally, through injection, by adherence of a medicated patch or other method. Further, a method of using fecal chymotrypsin level as a biomarker for the presence of a prion disease, or the likelihood of an individual to develop a prion disease is disclosed.
Owner:CUREMARK
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com