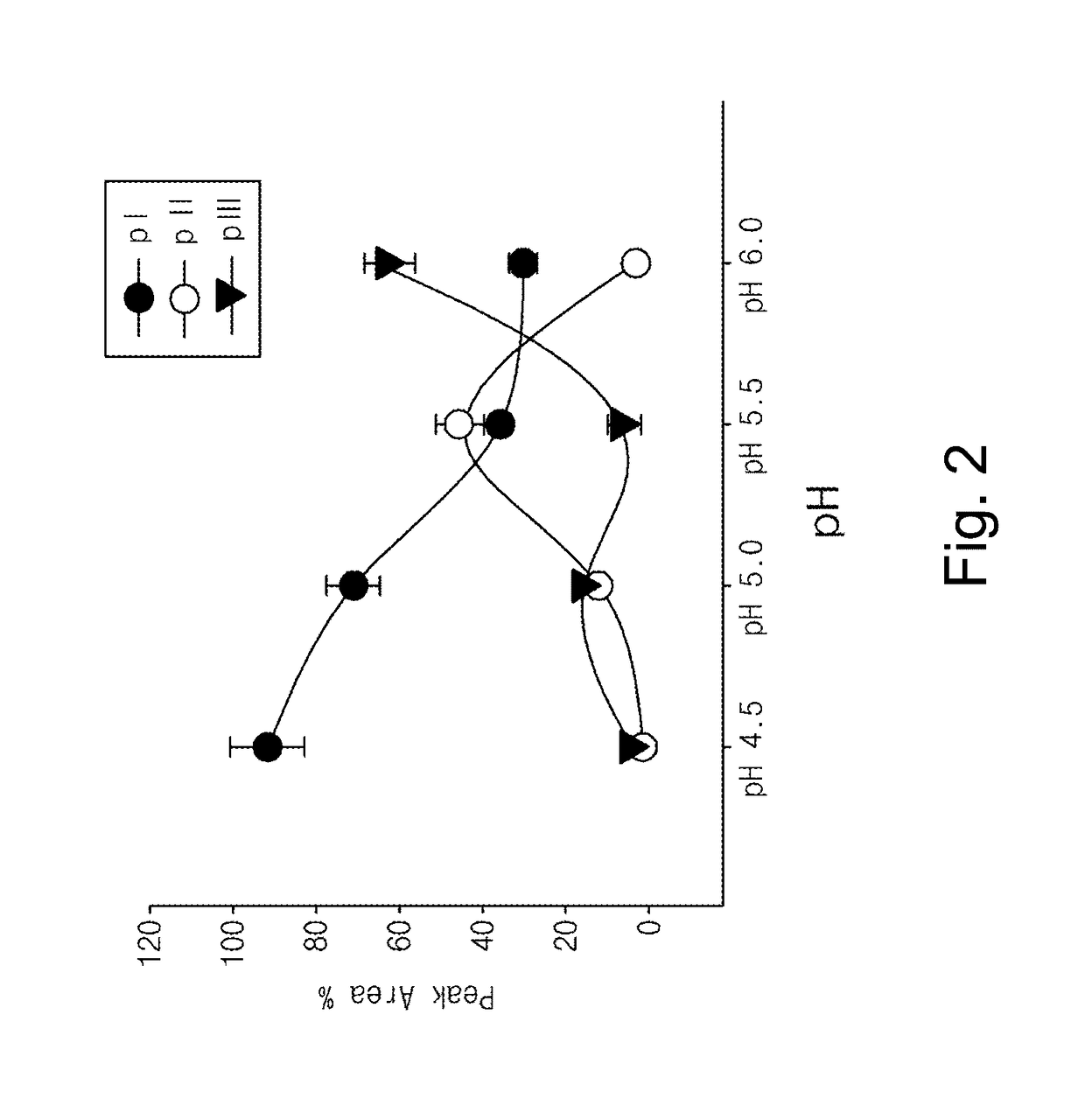
Patents
Literature
43 results about "Botulinum toxin type" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Injectable Botulinum Toxin Formulations
InactiveUS20100168023A1Low antigenicityExtended durationCosmetic preparationsSenses disorderClinical efficacyBotulinum toxin type
This invention provides novel injectable compositions comprising botulinum toxin that may be administered to a subject for various therapeutic, aesthetic and / or cosmetic purposes. The injectable compositions contemplated by the invention exhibit one or more advantages over conventional botulinum toxin formulations, including reduced antigenicity, a reduced tendency to undergo unwanted localized diffusion following injection, increased duration of clinical efficacy or enhanced potency relative, faster onset of clinical efficacy, and / or improved stability.
Owner:REVANCE THERAPEUTICS INC
Pharmaceutical botulinum toxin compositions
Owner:REVANCE THERAPEUTICS INC
Use of botulinum toxins for treating various disorders and conditions and associated pain
A method and composition for treating a patient suffering from a disease, disorder or condition and associated pain include the administration to the patient of a therapeutically effective amount of a neurotoxin selected from a group consisting of Botulinum toxin types A, B, C, D, E, F and G.
Owner:ALLERGAN INC
Botulinum toxin and the treatment of primary disorders of mood and affect
ActiveUS8926991B2Reduce transmissionRelieve symptomsBacterial antigen ingredientsPeptide/protein ingredientsBotulinum toxin typeClinical psychology
The invention provides methods for treating primary disorders of mood and affect, including depressive disorders, anxiety and sleep disorders and CNS disorders comprising the administration of a neurotoxin.
Owner:REVANCE THERAPEUTICS INC
Multiple botulinum toxins for treating neuromuscular disorders and conditions
InactiveUS20010021695A1Alleviation of muscle spasmsHelp with painNervous disorderPeptide/protein ingredientsDiseaseNeuromuscular disease
A method and composition for treating a patient suffering from a disease, disorder or condition include the administration to the patient of a therapeutically effective amount of a combination of at least two neurotoxins selected from a group consisting of botulinum toxin types A, B, C, D, E, F and G. The amount of each selected neurotoxin administered is further selected to control a duration of therapeutic activity of the administered combination.
Owner:ALLERGAN INC
High-Affinity Monoclonal Antibodies for Botulinum Toxin Type A
Method for treating a mucus secretion
InactiveUS6986893B2Relief the painHormone peptidesBacterial antigen ingredientsBotulinum toxin typeBotulinum toxin type G
A method and composition for treating a patient suffering from an excessive mucus secretion includes administration to the patient of a therapeutically effective amount of a botulinum toxin type A, B, C, D, E, F and / or G.
Owner:ALLERGAN INC
Liquid Product of Botulinum Toxin Type A
InactiveUS20120302507A1Easy to useConserves potencyHormone peptidesPeptide/protein ingredientsLiquid productCross infections
Disclosed are a liquid product of botulinum toxin type A and a method for conserving the potency of botulinum toxin type A using a dextrose solution. Free of a stabilizer, such as albumin or gelatin, the liquid product of botulinum toxin type A completely excludes the possibility of cross infections such as AIDS and bovine spongiform encephalopathy. In addition, botulinum toxin type A is preserved as a liquid product in combination with a dextrose solution and can be clinically used as is, without the aid of physiological saline. Therefore, the liquid product enjoys the advantage of being convenient for use and avoiding a decrease in the potency as occurs upon dilution with physiological saline. Serving as a natural preserving and stabilizing agent, the dextrose solution allows botulinum toxin type A to be stored and distributed in the form of liquid phase over a long period of time and conserves the potency of the toxin at a constant level, which in turn guarantees constant clinical results.
Owner:HAM JONG WOOK
Method of treating skin needing botulinum toxin type a treatment
InactiveUS20070154493A1Improve efficiencyExtended durationCosmetic preparationsBacterial antigen ingredientsBotulinum toxin typeCosmetic procedures
A treatment regimen for treating skin subject to a botulinum toxin type A cosmetic procedure involves the application of supplemental composition(s) such as preparatory composition(s), protective composition(s), and combinations thereof, and a corrective composition.
Owner:HATTENDORF JUDY +1
Composition for neutralizing botulinus toxin type-a, and human Anti-botulinus toxin type-a antibody
Provided herein is a means which is effective for botulism diseases and the prevention of the botulism diseases. Specifically provided is a plurality of human anti-botulinum toxin type-A antibodies having different epitopes from one another. Also specifically provided is a composition for neutralizing botulinum toxin type-A, which comprises a combination of two or more of the antibodies and which has a high neutralizing activity.
Owner:JAPAN AS REPRESENTED BY DIRECTOR GENERAL OF NAT INST OF INFECT IOUS DISEASES
Dose, localization, and formulation of botulinum toxins in skin and muscle
InactiveUS20140242110A1Simple and easily with dosingMinimize diffusionPeptide/protein ingredientsCarrier-bound antigen/hapten ingredientsDosing regimenSide effect
Formulations of and dosing protocols for the administration of botulinum toxin that maximize efficacy and specificity while minimizing the likelihood of overdosing and undesirable side effects of treatment. The formulations include positively charged carriers, such as cationic peptides, which otherwise have no inherent botulinum-toxin-like activity. The dosing regimen is based on the pattern, quantity, and location of neuromuscular junctions in the target tissue. Because the number of neuromuscular junctions in a target tissue remains generally stable throughout life and because the pharmacological effect of botulinum toxin is localized at the neuromuscular junction, dosing efficacy is unaffected by muscle mass, age of the patient, or body weight.
Owner:DT SCIMED
Implant for face filling and preparation method thereof
InactiveCN103007359AGood biocompatibilityImprove the lubrication effectProsthesisBotulinum toxin typeBiocompatibility Testing
The invention discloses an implant for face filling and a preparation method thereof. The implant for the face filling is a mixed suspended matter prepared by expanded polytetrafluoroethylene particles and a mixture, wherein the expanded polytetrafluoroethylene particles are obtained through the steps of: based on polytetrafluoroethylene as a raw material, expanding via extrusion and stretch, sintering, forming and cutting; the mixture contains sodium hyaluronate gel or botulinum toxin type A; and the mixed suspended matter can also contain antibiotics. The invention also provides the preparation method of the implant for the face filling. The implant for the face filling, prepared by adopting the method, can be implanted by adopting an injection way, is small in wound and convenient for forming, has good biocompatibility and has the advantages that through controlling sizes of micro pores in the expanded polytetrafluoroethylene particles, the histiocyte can grow in and the implant for the face filling can be permanently planted and cannot be absorbed by the body.
Owner:SHANGHAI SUOKANG MEDICAL IMPLANTS
Therapeutic use of botulinum toxins types A and B
A method of treating a patient suffering from a disease, disorder or condition includes the administration to the patient of a therapeutically effective amount of botulinum toxin of a selected serotype until the patient experiences loss of clinical response to the administered botulinum toxin and thereafter administering to the patient a therapeutically effective amount of another botulinum toxin of a different serotype.
Owner:AOKI K ROGER +3
Type a2 botulinum toxin preparation
InactiveUS20110033431A1Induce antibody productionTherapeutic effectBiocideBacteriaBotulinum toxin typeMedicine
A pharmaceutical preparation for use in a patient who has a neutralizing antibody to a botulinum toxin from type A1 Clostridium botulinum (type A1 botulinum toxin), said preparation comprising as an active ingredient 150 kDa type A neurotoxin from type A2 Clostridium botulinum (A2 NTX); a medicament for treating a disease with muscle overactivity for use in a patient who has a neutralizing antibody to a type A1 botulinum toxin, said medicament comprising as an active ingredient said A2 NTX; a method for treating a patient who has a neutralizing antibody to a type A1 botulinum toxin, said method comprising administering said A2 NTX to the patient; and a method for use of A2 NTX in a patient who has said neutralizing antibody. In accordance with the present invention, a problem can be solved of decrease in clinical response caused by a neutralizing antibody to a type A1 botulinum toxin produced when a patient is treated with a pharmaceutical preparation comprising a type A1 botulinum toxin.
Owner:JURIDICAL FOUND THE CHEMO SERO THERAPEUTIC RES INST
Methods for treating pain
Methods for treating pain by intrathecal administration to a human patient of a therapeutically effective amount of a neurotoxin such as botulinum toxin type A are disclosed.
Owner:ALLERGAN INC
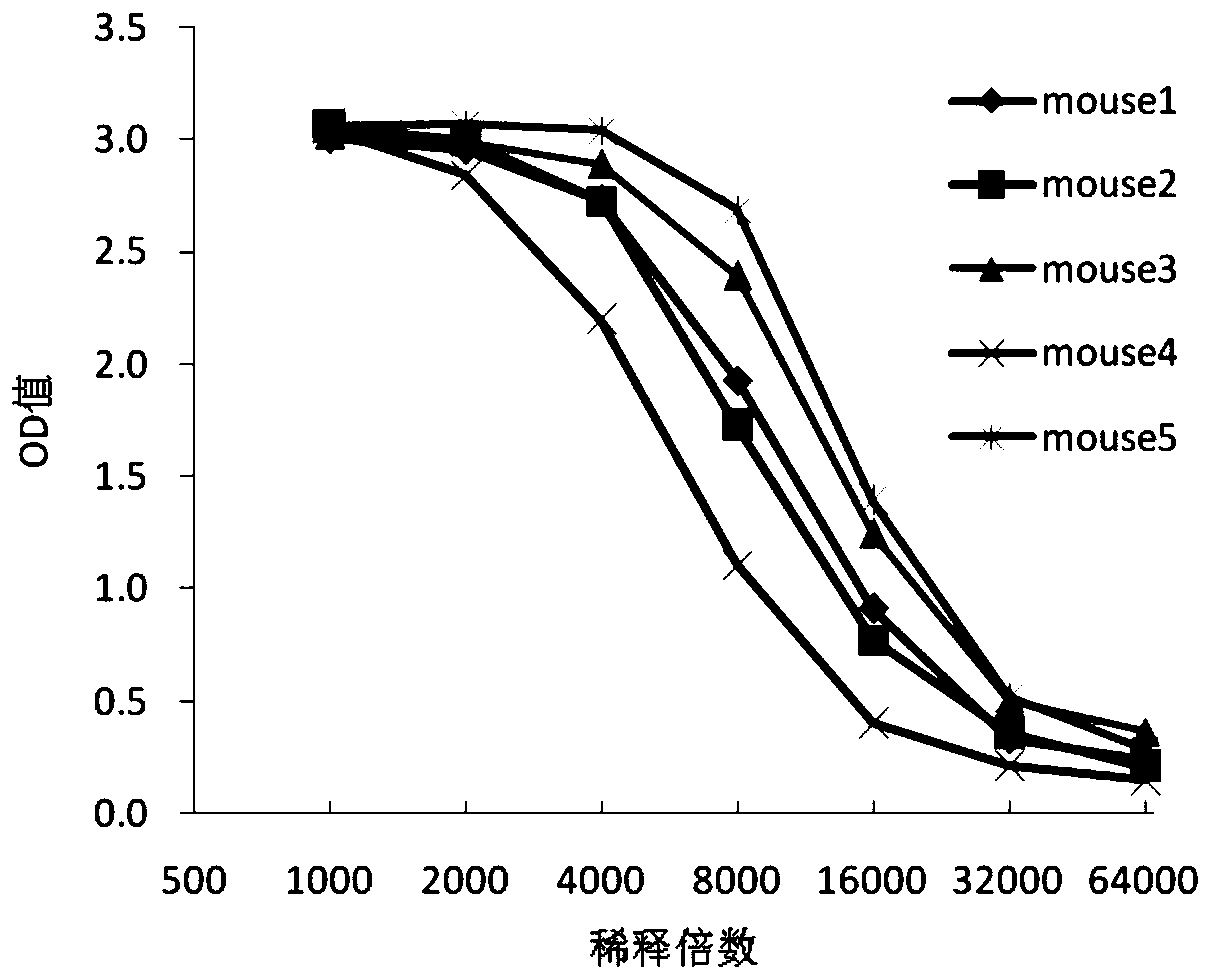
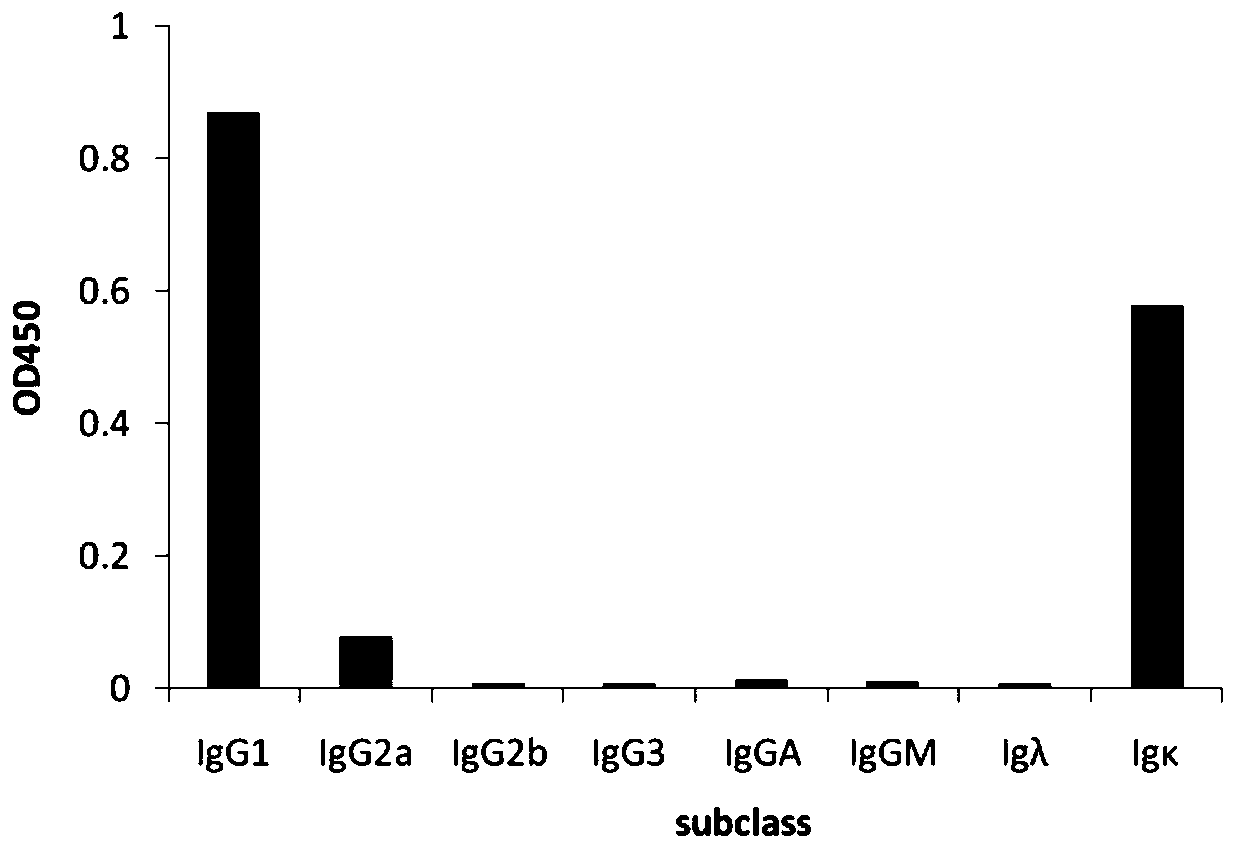
Mouse-derived monoclonal antibody for neutralizing botulinum toxin type A and application of mouse-derived monoclonal antibody
ActiveCN110317268APlay a partial protective effectAmplified geneAntibacterial agentsImmunoglobulins against bacteriaHeavy chainBotulinum toxin type
The invention relates to the technical field of biology, in particular to a mouse-derived monoclonal antibody for neutralizing botulinum toxin type A and application of the mouse-derived monoclonal antibody. The mouse-derived monoclonal antibody for neutralizing the botulinum toxin type A is composed of a light chain and a heavy chain; CDR1, CDR2 and CDR3 in a heavy chain variable region in the heavy chain are sequentially amino acid residues from the 71st site to the 94th site, amino acid residues from the 151st site to the 171st site and amino acid residues from the 286th site to the 315th site from the N terminal of a sequence 2 in a sequence table; CDR1, CDR2 and CDR3 in a light chain variable region in the light chain are sequentially amino acid residues from the 78th site to the 95thsite, amino acid residues from the 147th site to the 155th site and amino acid residues from the 259th site to the 282nd site from the N terminal of a sequence 4 in the sequence table.
Owner:ACADEMY OF MILITARY MEDICAL SCI
Medium composition for preparing botulinum toxin
Owner:DAEWOONG CO LTD
Composition for neutralizing botulinus toxin type-A, and human anti-botulinus toxin type-A antibody
Provided herein is a means which is effective for botulism diseases and the prevention of the botulism diseases. Specifically provided is a plurality of human anti-botulinum toxin type-A antibodies having different epitopes from one another. Also specifically provided is a composition for neutralizing botulinum toxin type-A, which comprises a combination of two or more of the antibodies and which has a high neutralizing activity.
Owner:JAPAN AS REPRESENTED BY DIRECTOR GENERAL OF NAT INST OF INFECT IOUS DISEASES
Non-diffusive botulinum toxin causing local muscle paralysis, and purification method thereof
InactiveUS20130071331A1Paralyzing muscleWay fastCompounds screening/testingBacterial antigen ingredientsPurification methodsMedicine
A method for purifying a non-spreading botulinum toxin that causes local muscle paralysis and a non-spreading botulinum toxin obtained thereby includes the steps of: subjecting a purified botulinum toxin type A product to ion-exchange chromatography using a controlled pH of buffer, concentration of sodium chloride (NaCl), thereby separating the botulinum toxin type A product into subfractions; and collecting a subfraction having an A260 / A280 value in a specific range from the separated subfractions.
Owner:MEDEXGEN
Neurotoxin therapy for postprandial hyperglycemia
InactiveUS20100104602A1Effectively treating obesityImpair gastricBacterial antigen ingredientsPeptide/protein ingredientsAcute hyperglycaemiaSmooth muscle
Botulinum toxin is increasingly being injected into visceral smooth muscle for a variety of indications. The present invention discloses intragastric administration of botulinum toxin to delay gastric emptying with the aim of inducing satiety and promoting weight-loss. The present invention also discloses the effects of intragastric administration of Botulinum toxin in reducing post-prandial hyperglycemia in patients suffering from Diabetes Mellitus.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Aerosolizable botulinum toxin type A AHc subunit vaccine dry powder inhalant
ActiveCN110327314AFacilitate depositionProlong contact reaction timeAntibacterial agentsPowder deliveryAntigenFreeze-drying
The invention discloses an aerosolizable botulinum toxin type A AHc subunit vaccine dry powder inhalant. The AHc subunit vaccine dry powder is successfully prepared by a spray freeze drying method. The mass median aerodynamic diameter (MMAD) is close to 2.5 [mu]m. The particles are spherical, and loose and porous in shape, and the distribution of particle size is uniform. The particle size of therange is beneficial to the deposition of the vaccine to the deep part of the lung, the contact reaction time between the antigen and the mucosa tissue is increased, and the immune protection effect isimproved. The recombinant AHc subunit vaccine dry powder prepared by the invention meets the requirements of lung delivery immunity, can protect mice against 1440 times LD<50> BoNT / A complex attackthrough lung delivery immunity, lays a foundation for the development of botulinum toxin subunit vaccine dry powder vaccine and the research of lung delivery immunity protection effect, and provides an advanced technical scheme for the development of inhalation dry powder vaccine.
Owner:ACADEMY OF MILITARY MEDICAL SCI
Method of isolating botulinum toxin from botulinum toxin-containing solution
ActiveUS11331598B2Effective isolationCation exchanger materialsHydrolasesBotulinum toxin typeOnabotulinum toxin
Provided is a method of isolating a botulinum toxin type A macro complex from a botulinum toxin-containing solution, the method including performing anion exchange chromatography and cation exchange chromatography.
Owner:MEDY TOX INC
Medium composition for preparing botulinum toxin
The present invention relates to a medium composition for production of botulinum toxin and, more particularly, to a medium composition for culture of Clostridium sp. capable of producing botulinum toxin. The medium composition of the present invention comprises at least one plant-derived peptone selected from the group consisting of a garden pea hydrolysate, a cotton seed hydrolysate and a wheat gluten hydrolysate. When the medium according to the present invention, which contains plant-derived peptones and minerals, is used for culture of Clostridium botulinum, the growth rate of the bacterium in the medium is about 1.5-2 times higher than that in the medium that is in current use. In addition, when botulinum toxin is produced by culturing the bacterium in the medium, infection with transmissible spongiform encephalopathy (TSE) or the like can be prevented by blocking introduction of animal-derived components.
Owner:DAEWOONG CO LTD
Non-diffusive botulinum toxin causing local muscle paralysis, and purification method thereof
InactiveUS9598683B2Paralyzing muscleWay fastCompounds screening/testingPeptide/protein ingredientsPurification methodsMedicine
A method for purifying a non-spreading botulinum toxin that causes local muscle paralysis and a non-spreading botulinum toxin obtained thereby includes the steps of: subjecting a purified botulinum toxin type A product to ion-exchange chromatography using a controlled pH of buffer, concentration of sodium chloride (NaCl), thereby separating the botulinum toxin type A product into subfractions; and collecting a subfraction having an A260 / A280 value in a specific range from the separated subfractions.
Owner:MEDEXGEN
Potency-extending agent of botulinum toxin type-a product
InactiveUS20160089440A1Increase the number ofBiocideInorganic non-active ingredientsBotulinum toxin typeDistilled water
A potency-extending agent of a botulinum toxin type-A product includes 1 ml of distilled water, 85 of dextrose, 4.28 mg of sodium chloride, and 10 mg of trehalose.
Owner:HAM JONG WOOK
Use of the neurotoxic component of a botulinum toxin for treating a spastic muscle
InactiveUS8187612B2Relief the painBacterial antigen ingredientsNervous disorderDiseaseBotulinum toxin type
A method and composition for treating a patient suffering from a disease, disorder or condition and associated pain include the administration to the patient of a therapeutically effective amount of a neurotoxin selected from a group consisting of botulinum toxin types A, B, C, D, E, F and G.
Owner:ALLERGAN INC
Botulinum toxin for primary disorders of mood and affect using neurotransmitter
The invention provides methods for treating primary disorders of mood and affect, including depressive disorders, anxiety, and sleep disorders and CNS disorders comprising the administration of a neurotoxin.
Owner:REVANCE THERAPEUTICS INC
Method of isolating botulinum toxin from botulinum toxin-containing solution
ActiveCN110099918AEfficient separationCation exchanger materialsHydrolasesBotulinum toxin typeOnabotulinum toxin
Provided is a method of isolating a botulinum toxin type A macro complex from a botulinum toxin-containing solution, the method including performing anion exchange chromatography and cation exchange chromatography.
Owner:MEDY TOX INC
Methods for treating pain and for treating a medication overuse disorder
InactiveCN1933849AImprove effectivenessBacterial antigen ingredientsNervous disorderAnalgesic rebound headacheCo administration
A headache can be treated more effectively by co-administration of a botulinum toxin and a triptan drug to a patient and / or the effectiveness of a triptan medication can be increased. The botulinum toxin can be botulinum toxin type A and the botulinum toxin can be administered to or to the vicinity of where a patient experiences or is predisposed to experience pain or a headache.
Owner:ALLERGAN INC
Botulinum toxin type a complex, and formulation thereof and usage method therefor
PendingUS20220257730A1Small molecular weightLow production costPowder deliveryCosmetic preparationsBotulinum toxin typeC. botulinum
The present invention relates to a botulinum toxin type A complex, and a formulation thereof and a usage method therefor. The present invention provides the botulinum toxin type A complex, comprising an HA70 component, and an HA17 component, an HA33 component, an NTNH component, and a BoNT / A1 component, wherein the botulinum toxin complex has a molecular weight of 740-790 kDa. Compared with the existing botulinum toxin complexes, the botulinum toxin complex of the present invention is smaller in molecular weight and higher in safety, and has a comparable treatment effect.
Owner:OBI PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com