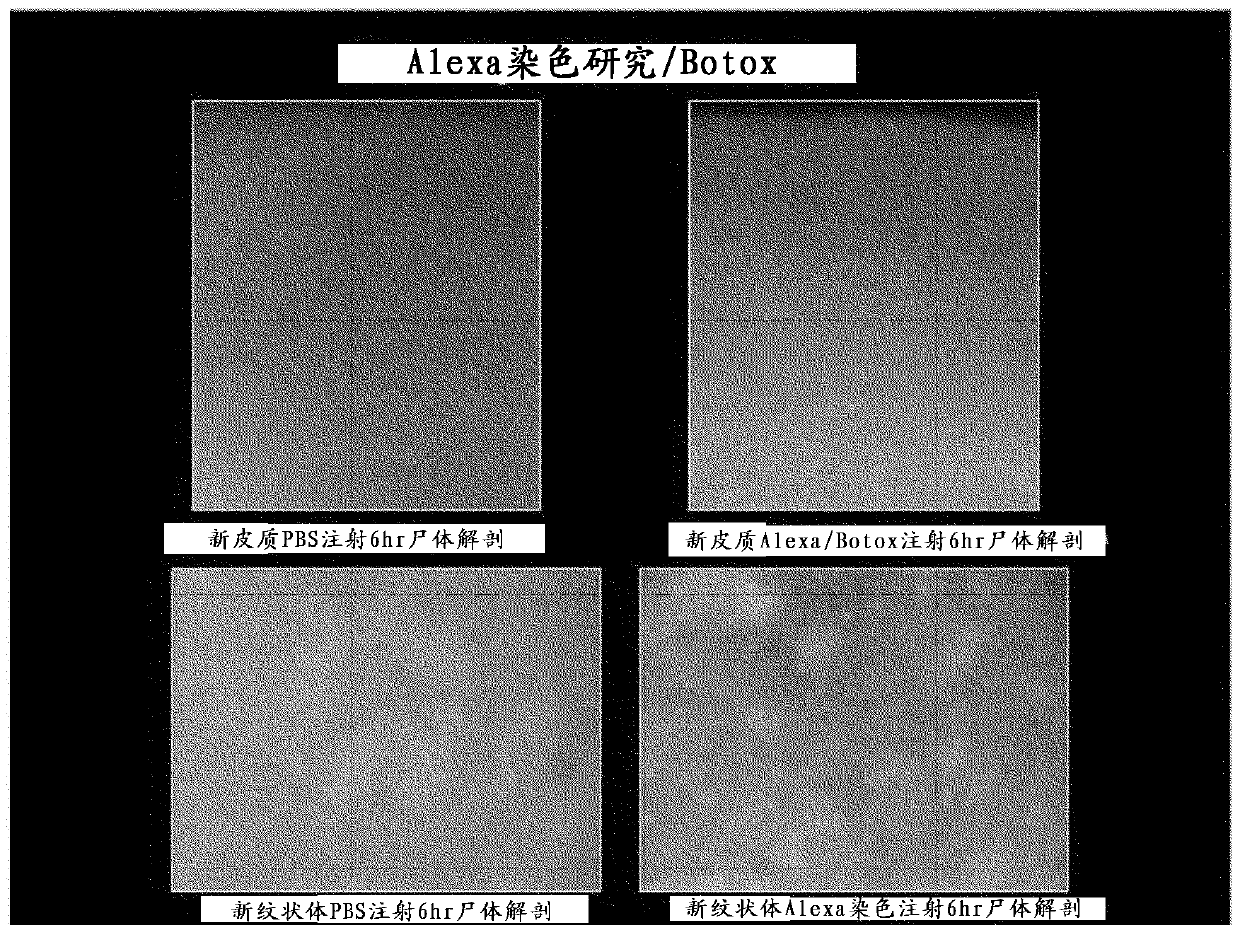
Botulinum toxin for primary disorders of mood and affect using neurotransmitter
A botulinum toxin and neurotransmitter technology, which is applied in neuromuscular system diseases, nervous system diseases, muscular system diseases, etc., can solve problems such as unexplainable and uncertain statistical significance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0224] A 78-year-old man with documented sleep disturbance and anxiety was initially diagnosed with blepharospasm. Subjects who administered botulinum toxin by injection recorded improved sleep and reduced anxiety.
Embodiment 2
[0226] A 44-year-old bus driver was diagnosed with hemifacial spasm and reported symptoms of anxiety. Botulinum toxin is administered by injection. Subjects recorded a better ability to cope with work-related stress and to cope with difficult situations with less stress.
Embodiment 3
[0228] Botulinum toxin administered by injection in the treatment of a 72-year-old consultant with a diagnosis of hemifacial spasm who reported sleep disturbance and anxiety. Subjects reported improved sleep and reduced anxiety and agitation.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com