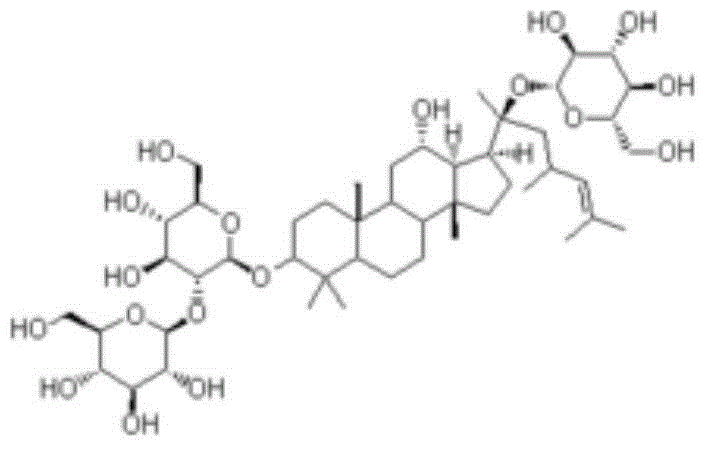
Novel application of ginsenoside Rd in preparing drug for preventing and/or treating microglial cell mediated diseases
A technology of microglia and ginsenosides, which can be used in drug combinations, muscular system diseases, nervous system diseases, etc., can solve the problems of no report of public ginsenoside Rd, no report of ginsenoside Rd microglia immunotoxicity, etc. , to achieve significant effect and high safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: the preparation of ginsenoside Rd
[0032] 1) Extract the Radix Notoginseng medicinal material with water, and concentrate the extract into a Radix Notoginseng extract containing 25% solids;
[0033] 2) After the Panax notoginseng extract is degreased, it is purified by D-type macroporous resin, washed with water: 95% ethanol according to the gradient elution method to remove triol glycoside components and most diol glycoside components outside Rd, and then separately with 70 % ethanol elutes the saponins mainly composed of ginsenoside Rd, collects the eluate and decolorizes it, recovers the solvent, concentrates it, and dries it under reduced pressure to make a dry product;
[0034] 3) The dry product was dissolved in 95.0% ethanol, and the insoluble matter was filtered out with a 50 μm filter membrane, and then absorbed by a monodisperse polymeric silica gel chromatography column, eluted and purified by gradient elution with water and 95.0% ethanol, and ...
Embodiment 2
[0036] Embodiment 2: the preparation of ginsenoside Rd
[0037] 1) Take ginseng and extract with ethanol with a concentration of 25%, recover the ethanol from the extract to obtain a Panax notoginseng extract containing 20% solids;
[0038] 2) After the ginseng extract is degreased, it is purified by D-type macroporous resin, washed with water: 95% ethanol according to the gradient elution method to remove the triol glycoside components and most of the diol glycoside components outside Rd, and then separately with 95% ethanol Ethanol elutes the saponins mainly composed of ginsenoside Rd, collects the eluate and decolorizes it, recovers the solvent, concentrates it, and dries it under reduced pressure to make a dry product;
[0039] 3) The dried product was dissolved in 96% ethanol, and the insoluble matter was filtered out with a 45 μm filter membrane, and then absorbed by a monodisperse polymeric silica gel chromatography column, eluted and purified by gradient elution with...
Embodiment 3
[0041] Embodiment 3: the preparation of ginsenoside Rd
[0042] 1) Extract the Radix Notoginseng medical material with 96% ethanol, recycle the ethanol from the extract to obtain a Radix Notoginseng extract containing 5% solids;
[0043]2) After the Panax notoginseng extract is degreased, it is purified by D-type macroporous resin, washed with water: 95% ethanol according to the gradient elution method to remove the triol glycoside components and most of the diol glycoside components outside Rd, and then separately with 95 % ethanol solution to elute the saponins mainly composed of ginsenoside Rd, collect the eluent and decolorize it, recover the solvent, concentrate, dry under reduced pressure, and make a dry product;
[0044] 3) The dried product was dissolved in 98.0% ethanol, and the insoluble matter was filtered out with a 1 μm filter membrane, and then absorbed by a monodisperse polymeric silica gel chromatography column, eluted and purified by gradient elution with wate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com